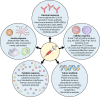
Factors That Influence the Immune Response to Vaccination
- PMID: 30867162
- PMCID: PMC6431125
- DOI: 10.1128/CMR.00084-18
Factors That Influence the Immune Response to Vaccination
Abstract
There is substantial variation between individuals in the immune response to vaccination. In this review, we provide an overview of the plethora of studies that have investigated factors that influence humoral and cellular vaccine responses in humans. These include intrinsic host factors (such as age, sex, genetics, and comorbidities), perinatal factors (such as gestational age, birth weight, feeding method, and maternal factors), and extrinsic factors (such as preexisting immunity, microbiota, infections, and antibiotics). Further, environmental factors (such as geographic location, season, family size, and toxins), behavioral factors (such as smoking, alcohol consumption, exercise, and sleep), and nutritional factors (such as body mass index, micronutrients, and enteropathy) also influence how individuals respond to vaccines. Moreover, vaccine factors (such as vaccine type, product, adjuvant, and dose) and administration factors (schedule, site, route, time of vaccination, and coadministered vaccines and other drugs) are also important. An understanding of all these factors and their impacts in the design of vaccine studies and decisions on vaccination schedules offers ways to improve vaccine immunogenicity and efficacy.
Keywords: antibodies; cellular; cytokines; humoral; immunization; immunoglobulin.
Copyright © 2019 American Society for Microbiology.
Figures
References
-
- World Health Organization. 2009. State of the world’s vaccines and immunization. WHO; Geneva, Switzerland.
-
- Anonymous. 1999. Impact of vaccines universally recommended for children—United States, 1990–1998. MMWR Morb Mortal Wkly Rep 48:243–248. - PubMed
-
- Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, Pirani A, Gernert K, Deng J, Marzolf B, Kennedy K, Wu H, Bennouna S, Oluoch H, Miller J, Vencio RZ, Mulligan M, Aderem A, Ahmed R, Pulendran B. 2009. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol 10:116–125. doi: 10.1038/ni.1688. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical