Clinical Implications of Circulating Tumor DNA Tumor Mutational Burden (ctDNA TMB) in Non-Small Cell Lung Cancer
- PMID: 30867242
- PMCID: PMC6656496
- DOI: 10.1634/theoncologist.2018-0433
Clinical Implications of Circulating Tumor DNA Tumor Mutational Burden (ctDNA TMB) in Non-Small Cell Lung Cancer
Abstract
Background: Tissue tumor mutational burden (TMB) has emerged as a potential biomarker predicting response to anti-programmed cell death-1 protein receptor (PD-1)/programmed cell death-1 protein ligand (PD-L1) therapy, but few studies have explored using circulating tumor DNA (ctDNA) TMB in non-small cell lung cancer (NSCLC).
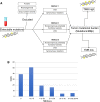
Materials and methods: A total of 136 patients with NSCLC with ctDNA testing were retrospectively evaluated from a single institution, along with a validation cohort from a second institution. ctDNA TMB was derived using the number of detected mutations over the DNA sequencing length.
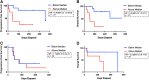
Results: Higher ctDNA TMB was significantly correlated with smoking history (p < .05, chi-squared test). Among patients treated with immune checkpoint inhibitors (n = 20), higher ctDNA TMB was significantly correlated with shorter progressive free survival (PFS) and overall survival (OS; 45 vs. 355 days; hazard ratio [HR], 5.6; 95% confidence interval [CI], 1.3-24.6; p < .01, and OS 106 days vs. not reached; HR, 6.0; 95% CI, 1.3-27.1; p < .01, respectively). In a small independent validation cohort (n = 12), there was a nonsignificant numerical difference for higher ctDNA TMB predicting shorter OS but not PFS. ctDNA TMB was not correlated with RECIST tumor burden estimation in the subset of patients treated with immune checkpoint blockade.
Conclusion: The findings indicate that higher ctDNA TMB, at the current commercial sequencing length, reflects worse clinical outcomes.
Implications for practice: Biomarkers to identify patients who will respond to immune checkpoint blockade are critical. Tissue tumor mutational burden (TMB) has emerged as a viable biomarker to predict response to anti-PD-1/PD-L1 therapy, but few studies have explored the meaning and potential clinical significance of noninvasive, blood-based TMB. Here, we investigated circulating tumor DNA (ctDNA) TMB and present data demonstrating that current ctDNA TMB may reflect tumor burden and that ctDNA panels with a greater number of mutations may be necessary to more accurately reflect tissue TMB.
Keywords: Circulating tumor DNA (ctDNA); NSCLC; PD‐1; PD‐L1; Tumor mutational burden (TMB).
© AlphaMed Press 2019.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures

Similar articles
-
Association of TP53 mutations with response and longer survival under immune checkpoint inhibitors in advanced non-small-cell lung cancer.Lung Cancer. 2019 Jun;132:65-71. doi: 10.1016/j.lungcan.2019.04.005. Epub 2019 Apr 8. Lung Cancer. 2019. PMID: 31097096
-
Assessment of Blood Tumor Mutational Burden as a Potential Biomarker for Immunotherapy in Patients With Non-Small Cell Lung Cancer With Use of a Next-Generation Sequencing Cancer Gene Panel.JAMA Oncol. 2019 May 1;5(5):696-702. doi: 10.1001/jamaoncol.2018.7098. JAMA Oncol. 2019. PMID: 30816954 Free PMC article.
-
Tumor mutational burden assessed by targeted NGS predicts clinical benefit from immune checkpoint inhibitors in non-small cell lung cancer.J Pathol. 2020 Jan;250(1):19-29. doi: 10.1002/path.5344. Epub 2019 Oct 24. J Pathol. 2020. PMID: 31471895 Free PMC article.
-
Biomarkers for immune checkpoint inhibition in non-small cell lung cancer (NSCLC).Cancer. 2020 Jan 15;126(2):260-270. doi: 10.1002/cncr.32468. Epub 2019 Nov 6. Cancer. 2020. PMID: 31691957 Free PMC article. Review.
-
Predictive biomarkers of response for immune checkpoint inhibitors in non-small-cell lung cancer.Eur J Cancer. 2019 Jan;106:144-159. doi: 10.1016/j.ejca.2018.11.002. Epub 2018 Dec 5. Eur J Cancer. 2019. PMID: 30528799 Review.
Cited by
-
Blood tumor mutation burden can predict the clinical response to immune checkpoint inhibitors in advanced non-small cell lung cancer patients.Cancer Immunol Immunother. 2021 Dec;70(12):3513-3524. doi: 10.1007/s00262-021-02943-2. Epub 2021 Apr 25. Cancer Immunol Immunother. 2021. PMID: 33899131 Free PMC article.
-
The Factors Predicting Concordant Epidermal Growth Factor Receptor (EGFR) Mutation Detected in Liquid/Tissue Biopsy and the Related Clinical Outcomes in Patients of Advanced Lung Adenocarcinoma with EGFR Mutations.J Clin Med. 2019 Oct 23;8(11):1758. doi: 10.3390/jcm8111758. J Clin Med. 2019. PMID: 31652678 Free PMC article.
-
Tumor Mutational Burden as a Predictive Biomarker for Response to Immune Checkpoint Inhibitors: A Review of Current Evidence.Oncologist. 2020 Jan;25(1):e147-e159. doi: 10.1634/theoncologist.2019-0244. Epub 2019 Oct 2. Oncologist. 2020. PMID: 31578273 Free PMC article. Review.
-
The Role of Cell-Free DNA in Cancer Treatment Decision Making.Cancers (Basel). 2022 Dec 12;14(24):6115. doi: 10.3390/cancers14246115. Cancers (Basel). 2022. PMID: 36551600 Free PMC article. Review.
-
On-treatment blood TMB as predictors for camrelizumab plus chemotherapy in advanced lung squamous cell carcinoma: biomarker analysis of a phase III trial.Mol Cancer. 2022 Jan 3;21(1):4. doi: 10.1186/s12943-021-01479-4. Mol Cancer. 2022. PMID: 34980131 Free PMC article. Clinical Trial.
References
-
- Jahr S, Hentze H, Englisch S et al. DNA fragments in the blood plasma of cancer patients: Quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res 2001;61:1659–1665. - PubMed
-
- Murtaza M, Dawson SJ, Tsui DW et al. Non‐invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature 2013;497:108–112. - PubMed
-
- Schwaederle M, Husain H, Fanta PT, et al. Use of liquid biopsies in clinical oncology: Pilot experience in 168 patients. Clin Cancer Res 2016;22:5497–5505. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

