The RNA-Protein Interactome of Differentiated Kidney Tubular Epithelial Cells
- PMID: 30867249
- PMCID: PMC6442340
- DOI: 10.1681/ASN.2018090914
The RNA-Protein Interactome of Differentiated Kidney Tubular Epithelial Cells
Erratum in
-
Correction: The RNA-Protein Interactome of Differentiated Kidney Tubular Epithelial Cells.J Am Soc Nephrol. 2025 Apr 11;36(7):1456. doi: 10.1681/ASN.0000000737. J Am Soc Nephrol. 2025. PMID: 40215110 Free PMC article. No abstract available.
Abstract
Background: RNA-binding proteins (RBPs) are fundamental regulators of cellular biology that affect all steps in the generation and processing of RNA molecules. Recent evidence suggests that regulation of RBPs that modulate both RNA stability and translation may have a profound effect on the proteome. However, regulation of RBPs in clinically relevant experimental conditions has not been studied systematically.
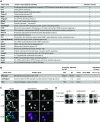
Methods: We used RNA interactome capture, a method for the global identification of RBPs to characterize the global RNA-binding proteome (RBPome) associated with polyA-tailed RNA species in murine ciliated epithelial cells of the inner medullary collecting duct. To study regulation of RBPs in a clinically relevant condition, we analyzed hypoxia-associated changes of the RBPome.
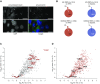
Results: We identified >1000 RBPs that had been previously found using other systems. In addition, we found a number of novel RBPs not identified by previous screens using mouse or human cells, suggesting that these proteins may be specific RBPs in differentiated kidney epithelial cells. We also found quantitative differences in RBP-binding to mRNA that were associated with hypoxia versus normoxia.
Conclusions: These findings demonstrate the regulation of RBPs through environmental stimuli and provide insight into the biology of hypoxia-response signaling in epithelial cells in the kidney. A repository of the RBPome and proteome in kidney tubular epithelial cells, derived from our findings, is freely accessible online, and may contribute to a better understanding of the role of RNA-protein interactions in kidney tubular epithelial cells, including the response of these cells to hypoxia.
Keywords: HIF; RBP; RNA-binding protein; cilia; hypoxia; tubule cells.
Copyright © 2019 by the American Society of Nephrology.
Figures




References
-
- Hentze MW, Castello A, Schwarzl T, Preiss T: A brave new world of RNA-binding proteins. Nat Rev Mol Cell Biol 19: 327–341, 2018 - PubMed
-
- Dutertre M, Vagner S: DNA-Damage Response RNA-Binding Proteins (DDRBPs): Perspectives from a new class of proteins and their RNA targets. J Mol Biol 429: 3139–3145, 2017 - PubMed
-
- Milek M, Landthaler M: Systematic detection of poly(A)+ RNA-interacting proteins and their differential binding. Methods Mol Biol 1649: 405–417, 2018 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

