Cell-to-Cell Spread Blocking Activity Is Extremely Limited in the Sera of Herpes Simplex Virus 1 (HSV-1)- and HSV-2-Infected Subjects
- PMID: 30867302
- PMCID: PMC6532082
- DOI: 10.1128/JVI.00070-19
Cell-to-Cell Spread Blocking Activity Is Extremely Limited in the Sera of Herpes Simplex Virus 1 (HSV-1)- and HSV-2-Infected Subjects
Abstract
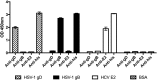
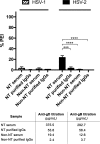
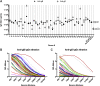
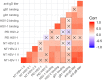
Herpes simplex virus 1 (HSV-1) and HSV-2 can evade serum antibody-mediated neutralization through cell-to-cell transmission mechanisms, which represent one of the central steps in disease reactivation. To address the role of humoral immunity in controlling HSV-1 and HSV-2 replication, we analyzed serum samples from 44 HSV-1 and HSV-2 seropositive subjects by evaluating (i) their efficiency in binding both the purified viral particles and recombinant gD and gB viral glycoproteins, (ii) their neutralizing activity, and (iii) their capacity to inhibit the cell-to-cell virus passage in vitro All of the sera were capable of binding gD, gB, and whole virions, and all sera significantly neutralized cell-free virus. However, neither whole sera nor purified serum IgG fraction was able to inhibit significantly cell-to-cell virus spreading in in vitro post-virus-entry infectious assays. Conversely, when spiked with an already described anti-gD human monoclonal neutralizing antibody capable of inhibiting HSV-1 and -2 cell-to-cell transmission, each serum boosted both its neutralizing and post-virus-entry inhibitory activity, with no interference exerted by serum antibody subpopulations.IMPORTANCE Despite its importance in the physiopathology of HSV-1 and -2 infections, the cell-to-cell spreading mechanism is still poorly understood. The data shown here suggest that infection-elicited neutralizing antibodies capable of inhibiting cell-to-cell virus spread can be underrepresented in most infected subjects. These observations can be of great help in better understanding the role of humoral immunity in controlling virus reactivation and in the perspective of developing novel therapeutic strategies, studying novel correlates of protection, and designing effective vaccines.
Keywords: cell-to-cell virus spread; herpes simplex virus; human monoclonal antibodies; humoral immunity; neutralizing activity; serum neutralizing antibodies.
Copyright © 2019 American Society for Microbiology.
Figures



Similar articles
-
Patient-Specific Neutralizing Antibody Responses to Herpes Simplex Virus Are Attributed to Epitopes on gD, gB, or Both and Can Be Type Specific.J Virol. 2015 Sep;89(18):9213-31. doi: 10.1128/JVI.01213-15. Epub 2015 Jun 24. J Virol. 2015. PMID: 26109729 Free PMC article.
-
Glycoprotein C of Herpes Simplex Virus 1 Shields Glycoprotein B from Antibody Neutralization.J Virol. 2020 Feb 14;94(5):e01852-19. doi: 10.1128/JVI.01852-19. Print 2020 Feb 14. J Virol. 2020. PMID: 31826995 Free PMC article.
-
Herpes simplex virus 1 envelope glycoprotein C shields glycoprotein D to protect virions from entry-blocking antibodies.J Virol. 2025 Apr 15;99(4):e0009025. doi: 10.1128/jvi.00090-25. Epub 2025 Mar 26. J Virol. 2025. PMID: 40135897 Free PMC article.
-
The Roles of Host and Viral Antibody Fc Receptors in Herpes Simplex Virus (HSV) and Human Cytomegalovirus (HCMV) Infections and Immunity.Front Immunol. 2019 Sep 6;10:2110. doi: 10.3389/fimmu.2019.02110. eCollection 2019. Front Immunol. 2019. PMID: 31555298 Free PMC article. Review.
-
Recent advances in vaccine development for herpes simplex virus types I and II.Hum Vaccin Immunother. 2013 Apr;9(4):729-35. doi: 10.4161/hv.23289. Epub 2013 Feb 26. Hum Vaccin Immunother. 2013. PMID: 23442925 Free PMC article. Review.
Cited by
-
B cells, antibody-secreting cells, and virus-specific antibodies respond to herpes simplex virus 2 reactivation in skin.J Clin Invest. 2021 May 3;131(9):e142088. doi: 10.1172/JCI142088. J Clin Invest. 2021. PMID: 33784252 Free PMC article. Clinical Trial.
-
Mass Spectrometric Characterization of HSV-1 L-Particles From Human Dendritic Cells and BHK21 Cells and Analysis of Their Functional Role.Front Microbiol. 2020 Sep 29;11:1997. doi: 10.3389/fmicb.2020.01997. eCollection 2020. Front Microbiol. 2020. PMID: 33117298 Free PMC article.
-
Ancient herpes simplex 1 genomes reveal recent viral structure in Eurasia.Sci Adv. 2022 Jul 29;8(30):eabo4435. doi: 10.1126/sciadv.abo4435. Epub 2022 Jul 27. Sci Adv. 2022. PMID: 35895820 Free PMC article.
-
Effector functions are required for broad and potent protection of neonatal mice with antibodies targeting HSV glycoprotein D.Cell Rep Med. 2024 Feb 20;5(2):101417. doi: 10.1016/j.xcrm.2024.101417. Epub 2024 Feb 12. Cell Rep Med. 2024. PMID: 38350452 Free PMC article.
-
Extracellular vesicles originating from autophagy mediate an antibody-resistant spread of classical swine fever virus in cell culture.Autophagy. 2022 Jun;18(6):1433-1449. doi: 10.1080/15548627.2021.1987673. Epub 2021 Nov 5. Autophagy. 2022. PMID: 34740307 Free PMC article.
References
-
- Corey L, Langenberg AGM, Ashley R, Sekulovich RE, Izu AE, Douglas JM Jr, Handsfield HH, Warren T, Marr L, Tyring S, DiCarlo R, Adimora AA, Leone P, Dekker CL, Burke RL, Leong WP, Straus SE. 1999. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. JAMA 282:331–340. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

