Design and Characterization of Cholesterylated Peptide HIV-1/2 Fusion Inhibitors with Extremely Potent and Long-Lasting Antiviral Activity
- PMID: 30867304
- PMCID: PMC6532087
- DOI: 10.1128/JVI.02312-18
Design and Characterization of Cholesterylated Peptide HIV-1/2 Fusion Inhibitors with Extremely Potent and Long-Lasting Antiviral Activity
Abstract
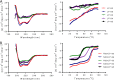
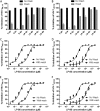
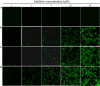
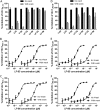
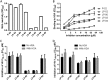
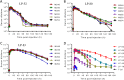
HIV infection requires lifelong treatment with multiple antiretroviral drugs in a combination, which ultimately causes cumulative toxicities and drug resistance, thus necessitating the development of novel antiviral agents. We recently found that enfuvirtide (T-20)-based lipopeptides conjugated with fatty acids have dramatically increased in vitro and in vivo anti-HIV activities. Herein, a group of cholesterol-modified fusion inhibitors were characterized with significant findings. First, novel cholesterylated inhibitors, such as LP-83 and LP-86, showed the most potent activity in inhibiting divergent human immunodeficiency virus type 1 (HIV-1), HIV-2, and simian immunodeficiency virus (SIV). Second, the cholesterylated inhibitors were highly active to inhibit T-20-resistant mutants that still conferred high resistance to the fatty acid derivatives. Third, the cholesterylated inhibitors had extremely potent activity to block HIV envelope (Env)-mediated cell-cell fusion, especially a truncated minimum lipopeptide (LP-95), showing a greatly increased potency relative to its inhibition on virus infection. Fourth, the cholesterylated inhibitors efficiently bound to both the cellular and viral membranes to exert their antiviral activities. Fifth, the cholesterylated inhibitors displayed low cytotoxicity and binding capacity with human serum albumin. Sixth, we further demonstrated that LP-83 exhibited extremely potent and long-lasting anti-HIV activity in rhesus monkeys. Taken together, the present results help our understanding on the mechanism of action of lipopeptide-based viral fusion inhibitors and facilitate the development of novel anti-HIV drugs.IMPORTANCE The peptide drug enfuvirtide (T-20) remains the only membrane fusion inhibitor available for treatment of viral infection, which is used in combination therapy of HIV-1 infection; however, it exhibits relatively low antiviral activity and a genetic barrier to inducing resistance, calling for the continuous development for novel anti-HIV agents. In this study, we report cholesterylated fusion inhibitors showing the most potent and broad anti-HIV activities to date. The new inhibitors have been comprehensively characterized for their modes of action and druggability, including small size, low cytotoxicity, binding ability to human serum albumin (HSA), and, especially, extremely potent and long-lasting antiviral activity in rhesus monkeys. Therefore, the present studies have provided new drug candidates for clinical development, which can also be used as tools to probe the mechanisms of viral entry and inhibition.
Keywords: HIV-1; HIV-2; T-20; fusion inhibitor; lipopeptide.
Copyright © 2019 American Society for Microbiology.
Figures

Similar articles
-
A Lipopeptide HIV-1/2 Fusion Inhibitor with Highly Potent In Vitro, Ex Vivo, and In Vivo Antiviral Activity.J Virol. 2017 May 12;91(11):e00288-17. doi: 10.1128/JVI.00288-17. Print 2017 Jun 1. J Virol. 2017. PMID: 28356533 Free PMC article.
-
Structural and Functional Characterization of Membrane Fusion Inhibitors with Extremely Potent Activity against Human Immunodeficiency Virus Type 1 (HIV-1), HIV-2, and Simian Immunodeficiency Virus.J Virol. 2018 Sep 26;92(20):e01088-18. doi: 10.1128/JVI.01088-18. Print 2018 Oct 15. J Virol. 2018. PMID: 30089693 Free PMC article.
-
Design of Novel HIV-1/2 Fusion Inhibitors with High Therapeutic Efficacy in Rhesus Monkey Models.J Virol. 2018 Jul 31;92(16):e00775-18. doi: 10.1128/JVI.00775-18. Print 2018 Aug 15. J Virol. 2018. PMID: 29899103 Free PMC article.
-
HIV entry and fusion inhibitors.Expert Opin Emerg Drugs. 2004 May;9(1):1-7. doi: 10.1517/eoed.9.1.1.32950. Expert Opin Emerg Drugs. 2004. PMID: 15155132 Review.
-
Resistance to enfuvirtide, the first HIV fusion inhibitor.J Antimicrob Chemother. 2004 Aug;54(2):333-40. doi: 10.1093/jac/dkh330. Epub 2004 Jul 1. J Antimicrob Chemother. 2004. PMID: 15231762 Review.
Cited by
-
Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy.Signal Transduct Target Ther. 2021 Jun 11;6(1):233. doi: 10.1038/s41392-021-00653-w. Signal Transduct Target Ther. 2021. PMID: 34117216 Free PMC article. Review.
-
The Tryptophan-Rich Motif of HIV-1 gp41 Can Interact with the N-Terminal Deep Pocket Site: New Insights into the Structure and Function of gp41 and Its Inhibitors.J Virol. 2019 Dec 12;94(1):e01358-19. doi: 10.1128/JVI.01358-19. Print 2019 Dec 12. J Virol. 2019. PMID: 31619552 Free PMC article.
-
Design of a Bispecific HIV Entry Inhibitor Targeting the Cell Receptor CD4 and Viral Fusion Protein Gp41.Front Cell Infect Microbiol. 2022 May 27;12:916487. doi: 10.3389/fcimb.2022.916487. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35711654 Free PMC article.
-
Conserved Residue Asn-145 in the C-Terminal Heptad Repeat Region of HIV-1 gp41 is Critical for Viral Fusion and Regulates the Antiviral Activity of Fusion Inhibitors.Viruses. 2019 Jul 3;11(7):609. doi: 10.3390/v11070609. Viruses. 2019. PMID: 31277353 Free PMC article.
-
Pan-coronavirus fusion inhibitors possess potent inhibitory activity against HIV-1, HIV-2, and simian immunodeficiency virus.Emerg Microbes Infect. 2021 Dec;10(1):810-821. doi: 10.1080/22221751.2021.1917309. Emerg Microbes Infect. 2021. PMID: 33847245 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

