Acquired von Willebrand Syndrome Associated with Cardiovascular Diseases
- PMID: 30867356
- PMCID: PMC6456452
- DOI: 10.5551/jat.RV17031
Acquired von Willebrand Syndrome Associated with Cardiovascular Diseases
Abstract
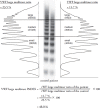
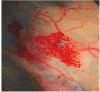
The blood glycoprotein von Willebrand factor (VWF) plays an important role in hemostasis and thrombosis.VWF is produced and secreted as large multimers by endothelial cells and megakaryocytes. It is then cleaved in a sheer-stress dependent manner by a specific protease, ADAMTS13, into multimers consisting of 2-80 subunits. Among VWF multimers, high molecular weight (HMW) multimers play important roles in platelet aggregation. Therefore, their loss induces a hemostatic disorder known as von Willebrand disease (VWD) type 2A. Various cardiovascular diseases, such as aortic stenosis, hypertrophic obstructive cardiomyopathy (HOCM), and several congenital structural diseases, as well as mechanical circulatory support systems, generate excessive high shear stress in the bloodstream. These cause excessive cleavage of VWF multimers resulting in a loss of HMW multimers, known as acquired von Willebrand syndrome (AVWS), a hemostatic disorder similar to VWD type 2A. Bleeding often occurs in the gastrointestinal tract since a fragile angiodysplasia develops associated with these diseases. Radical treatment for AVWS is to remove the pathological high shear causing AVWS.
Keywords: Acquired von Willebrand’s syndrome; Aortic stenosis; ECMO; Heyde’s syndrome; Left ventricular assist device; von Willebrand factor.
Figures


References
-
- Sadler JE: Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem, 1998; 67: 395-424 - PubMed
-
- Rondaij MG, Bierings R, Kragt A, van Mourik JA, Voorberg J: Dynamics and plasticity of Weibel-Palade bodies in endothelial cells. Arterioscler Thromb Vasc Biol, 2006; 26: 1002-1007 - PubMed
-
- El-Sayed MS, Sale C, Jones PG, Chester M: Blood hemostasis in exercise and training. Med Sci Sports Exerc, 2000; 32: 918-925 - PubMed
-
- Pottinger BE, Read RC, Paleolog EM, Higgins PG, Pearson JD: von Willebrand factor is an acute phase reactant in man. Throm Res, 1989; 53: 387-394 - PubMed

