Contractile forces in platelet aggregates under microfluidic shear gradients reflect platelet inhibition and bleeding risk
- PMID: 30867419
- PMCID: PMC6416331
- DOI: 10.1038/s41467-019-09150-9
Contractile forces in platelet aggregates under microfluidic shear gradients reflect platelet inhibition and bleeding risk
Abstract
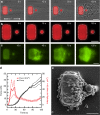
Platelets contract forcefully after their activation, contributing to the strength and stability of platelet aggregates and fibrin clots during blood coagulation. Viscoelastic approaches can be used to assess platelet-induced clot strengthening, but they require thrombin and fibrin generation and are unable to measure platelet forces directly. Here, we report a rapid, microfluidic approach for measuring the contractile force of platelet aggregates for the detection of platelet dysfunction. We find that platelet forces are significantly reduced when blood samples are treated with inhibitors of myosin, GPIb-IX-V, integrin αIIbβ3, P2Y12, or thromboxane generation. Clinically, we find that platelet forces are measurably lower in cardiology patients taking aspirin. We also find that measuring platelet forces can identify Emergency Department trauma patients who subsequently require blood transfusions. Together, these findings indicate that microfluidic quantification of platelet forces may be a rapid and useful approach for monitoring both antiplatelet therapy and traumatic bleeding risk.
Conflict of interest statement
N.J.S., N.J.W., L.H.T, A.K., A.O.S., S.F. and N.T. are shareholders, consultants, and/or employees of Stasys Medical Corp., a company created to develop the platelet force technology for clinical use. Two US patents have been filed covering the technology: US 9,213,024 B2 (Microfluidic devices for measuring platelet coagulation and associated systems and methods) and US 9,140,684 B2 (Device to expose cells to fluid shear forces and associated systems and methods). E.L., A.S.J., X.W. and T.R. declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

