Poly(ADP-ribose) polymerase 1 accelerates vascular calcification by upregulating Runx2
- PMID: 30867423
- PMCID: PMC6416341
- DOI: 10.1038/s41467-019-09174-1
Poly(ADP-ribose) polymerase 1 accelerates vascular calcification by upregulating Runx2
Abstract
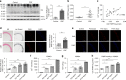
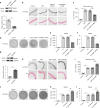
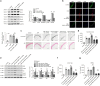
Vascular calcification is highly prevalent in end-stage renal diseases and is predictive of cardiovascular events and mortality. Poly(ADP-ribose) polymerase 1 (PARP1) inhibition or deletion is vasoprotective in several disease models. Here we show that PARP activity is increased in radial artery samples from patients with chronic renal failure, in arteries from uraemic rats, and in calcified vascular smooth muscle cells (VSMCs) in vitro. PARP1 deficiency blocks, whereas PARP1 overexpression exacerbates, the transdifferentiation of VSMCs from a contractile to an osteogenic phenotype, the expression of mineralization-regulating proteins, and calcium deposition. PARP1 promotes Runx2 expression, and Runx2 deficiency offsets the pro-calcifying effects of PARP1. Activated PARP1 suppresses miRNA-204 expression via the IL-6/STAT3 pathway and thus relieves the repression of its target, Runx2, resulting in increased Runx2 protein. Together, these results suggest that PARP1 counteracts vascular calcification and that therapeutic agents that influence PARP1 activity may be of benefit to treat vascular calcification.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

