Field transcriptome revealed a novel relationship between nitrate transport and flowering in Japanese beech
- PMID: 30867453
- PMCID: PMC6416253
- DOI: 10.1038/s41598-019-39608-1
Field transcriptome revealed a novel relationship between nitrate transport and flowering in Japanese beech
Abstract
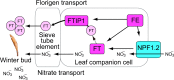
Recent advances in molecular and genetic studies about flowering time control have been increasingly available to elucidate the physiological mechanism underlying masting, the intermittent and synchronized production of a large amount of flowers and seeds in plant populations. To identify unexplored developmental and physiological processes associated with masting, genome-wide transcriptome analysis is a promising tool, but such analyses have yet to be performed. We established a field transcriptome using a typical masting species, Japanese beech (Fagus crenata Blume), over two years, and analyzed the data using a nonlinear time-series analysis called convergent cross mapping. Our field transcriptome was found to undergo numerous changes depending on the status of floral induction and season. An integrated approach of high-throughput transcriptomics and causal inference was successful at detecting novel causal regulatory relationships between nitrate transport and florigen synthesis/transport in a forest tree species. The synergistic activation of nitrate transport and floral transition could be adaptive to simultaneously satisfy floral transition at the appropriate timing and the nitrogen demand needed for flower formation.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Silvertown JW. The evolutionary ecology of mast seeding in trees. Biol. J. Linn. Soc. 1980;14:235–250. doi: 10.1111/j.1095-8312.1980.tb00107.x. - DOI
-
- Kelly D, Sork VL. Mast Seeding in Perennial Plants: Why, How, Where? Annu. Rev. Ecol. Syst. 2002;33:427–447. doi: 10.1146/annurev.ecolsys.33.020602.095433. - DOI
-
- Monselise, S. P. & Goldschmidt, E. E. Alternate bearing in fruit trees. Hortic. Rev. (Am. Soc. Hortic. Sci). 4, 10.1002/9781118060773.ch5 (1982).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

