In-silico design of a multi-epitope vaccine candidate against onchocerciasis and related filarial diseases
- PMID: 30867498
- PMCID: PMC6416346
- DOI: 10.1038/s41598-019-40833-x
In-silico design of a multi-epitope vaccine candidate against onchocerciasis and related filarial diseases
Abstract
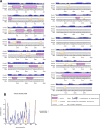
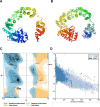
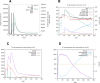
Onchocerciasis is a parasitic disease with high socio-economic burden particularly in sub-Saharan Africa. The elimination plan for this disease has faced numerous challenges. A multi-epitope prophylactic/therapeutic vaccine targeting the infective L3 and microfilaria stages of the parasite's life cycle would be invaluable to achieve the current elimination goal. There are several observations that make the possibility of developing a vaccine against this disease likely. For example, despite being exposed to high transmission rates of infection, 1 to 5% of people have no clinical manifestations of the disease and are thus considered as putatively immune individuals. An immuno-informatics approach was applied to design a filarial multi-epitope subunit vaccine peptide consisting of linear B-cell and T-cell epitopes of proteins reported to be potential novel vaccine candidates. Conservation of the selected proteins and predicted epitopes in other parasitic nematode species suggests that the generated chimera could be helpful for cross-protection. The 3D structure was predicted, refined, and validated using bioinformatics tools. Protein-protein docking of the chimeric vaccine peptide with the TLR4 protein predicted efficient binding. Immune simulation predicted significantly high levels of IgG1, T-helper, T-cytotoxic cells, INF-γ, and IL-2. Overall, the constructed recombinant putative peptide demonstrated antigenicity superior to current vaccine candidates.
Conflict of interest statement
The authors declare no competing interests.
Figures


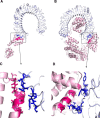

References
-
- Vlaminck J, Fischer PU, Weil GJ. Diagnostic Tools for Onchocerciasis Elimination Programs. Trends Parasitol. 2015;31:571–82. - PubMed
-
- Pion SD, Kamgno J, Demanga N, Boussinesq M. Excess mortality associated with blindness in the onchocerciasis focus of the Mbam Valley, Cameroon. Ann Trop Med Parasitol. 2002;96:181–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

