Identification of fibrinogen as a natural inhibitor of MMP-2
- PMID: 30867536
- PMCID: PMC6416301
- DOI: 10.1038/s41598-019-40983-y
Identification of fibrinogen as a natural inhibitor of MMP-2
Abstract
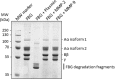
Non-genetic MMP-2 insufficiency is a relatively unexplored condition which could be induced by pathological overexpression of endogenous MMP-2 inhibitors such as TIMPs and/or the acute phase reactant alpha-2-macroglobulin. Here, we investigate the hypothesis that human fibrinogen (FBG) - an acute phase reactant - inhibits human MMP-2. Following an unexpected observation where sera from human donors including arthritis patients with increased levels of serum FBG exhibited reduced binding of serum proMMP-2 to gelatin, we found that human FBG (0 to 3.6 mg/mL i.e., 0 to 10.6 μM) concentration-dependently inhibited human proMMP-2 and MMP2 from binding to gelatin. Moreover, at normal physiological concentrations, FBG (5.29-11.8 μM) concentration-dependently inhibited (40-70% inhibition) the cleavage of fluorescein-conjugated gelatin by MMP-2, but not MMP-9. Indicative of a mixed-type (combination of competitive and non-competitive) inhibition mechanism, FBG reduced the Vmax (24.9 ± 0.7 min-1 to 17.7 ± 0.9 min-1, P < 0.05) and increased the Michaelis-Menten constant KM (204 ± 6 nM to 478 ± 50 nM, P < 0.05) for the reaction of MMP-2 cleavage of fluorescein-conjugated gelatin. In silico analyses and studies of FBG neutralization with anti-FBG antibodies implicated the domains D and E of FBG in the inhibition of MMP-2. In conclusion, FBG is a natural selective MMP-2 inhibitor, whose pathological elevation could lead to MMP-2 insufficiency in humans.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
E. coli expression of TIMP-4 and comparative kinetic studies with TIMP-1 and TIMP-2: insights into the interactions of TIMPs and matrix metalloproteinase 2 (gelatinase A).Biochemistry. 2002 Dec 17;41(50):15025-35. doi: 10.1021/bi026454l. Biochemistry. 2002. PMID: 12475252
-
Purification and characterization of matrix metalloproteinase 9 from U937 monocytic leukaemia and HT1080 fibrosarcoma cells.Biochem J. 1992 Jul 15;285 ( Pt 2)(Pt 2):603-11. doi: 10.1042/bj2850603. Biochem J. 1992. PMID: 1379048 Free PMC article.
-
Expression of matrix metalloproteinase-2 and -9 in human ligamentum flavum cells treated with tumor necrosis factor-α and interleukin-1β.J Neurosurg Spine. 2016 Mar;24(3):428-35. doi: 10.3171/2015.6.SPINE141271. Epub 2015 Nov 13. J Neurosurg Spine. 2016. PMID: 26565765
-
Matrix metalloproteinase and tissue inhibitors of metalloproteinase secretion by haematopoietic and stromal precursors and their production in normal and leukaemic long-term marrow cultures.Br J Haematol. 2001 Dec;115(3):595-604. doi: 10.1046/j.1365-2141.2001.03160.x. Br J Haematol. 2001. PMID: 11736941
-
Tumors and fibrinogen. The role of fibrinogen as an extracellular matrix protein.Ann N Y Acad Sci. 2001;936:406-25. Ann N Y Acad Sci. 2001. PMID: 11460495 Review.
Cited by
-
Corneal proteome and differentially expressed corneal proteins in highly myopic chicks using a label-free SWATH-MS quantification approach.Sci Rep. 2021 Mar 9;11(1):5495. doi: 10.1038/s41598-021-84904-4. Sci Rep. 2021. PMID: 33750851 Free PMC article.
-
A Fibrinogen Alpha Fragment Mitigates Chemotherapy-Induced MLL Rearrangements.Front Oncol. 2021 Jun 18;11:689063. doi: 10.3389/fonc.2021.689063. eCollection 2021. Front Oncol. 2021. PMID: 34222016 Free PMC article.
-
Evaluating the efficacy of extracted squalene from seed oil in the form of microemulsion for the treatment of COVID-19: A clinical study.J Med Virol. 2022 Jan;94(1):119-130. doi: 10.1002/jmv.27273. Epub 2021 Aug 26. J Med Virol. 2022. PMID: 34403141 Free PMC article. Clinical Trial.
-
Apolipoprotein-A1 transports and regulates MMP2 in the blood.Nat Commun. 2025 Apr 22;16(1):3752. doi: 10.1038/s41467-025-59062-0. Nat Commun. 2025. PMID: 40263360 Free PMC article.
-
Inhibition of MMP2-PEX by a novel ester of dihydroxy cinnamic and linoleic acid from the seagrass Cymodocea serrulata.Sci Rep. 2021 Jun 1;11(1):11451. doi: 10.1038/s41598-021-90845-9. Sci Rep. 2021. PMID: 34075089 Free PMC article.
References
-
- McQuibban GA, et al. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood. 2002;100:1160–1167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

