Histone H3 trimethylation at lysine 36 guides m6A RNA modification co-transcriptionally
- PMID: 30867593
- PMCID: PMC6438714
- DOI: 10.1038/s41586-019-1016-7
Histone H3 trimethylation at lysine 36 guides m6A RNA modification co-transcriptionally
Abstract
DNA and histone modifications have notable effects on gene expression1. Being the most prevalent internal modification in mRNA, the N6-methyladenosine (m6A) mRNA modification is as an important post-transcriptional mechanism of gene regulation2-4 and has crucial roles in various normal and pathological processes5-12. However, it is unclear how m6A is specifically and dynamically deposited in the transcriptome. Here we report that histone H3 trimethylation at Lys36 (H3K36me3), a marker for transcription elongation, guides m6A deposition globally. We show that m6A modifications are enriched in the vicinity of H3K36me3 peaks, and are reduced globally when cellular H3K36me3 is depleted. Mechanistically, H3K36me3 is recognized and bound directly by METTL14, a crucial component of the m6A methyltransferase complex (MTC), which in turn facilitates the binding of the m6A MTC to adjacent RNA polymerase II, thereby delivering the m6A MTC to actively transcribed nascent RNAs to deposit m6A co-transcriptionally. In mouse embryonic stem cells, phenocopying METTL14 knockdown, H3K36me3 depletion also markedly reduces m6A abundance transcriptome-wide and in pluripotency transcripts, resulting in increased cell stemness. Collectively, our studies reveal the important roles of H3K36me3 and METTL14 in determining specific and dynamic deposition of m6A in mRNA, and uncover another layer of gene expression regulation that involves crosstalk between histone modification and RNA methylation.
Figures






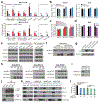
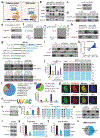
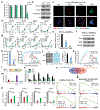

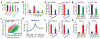


Comment in
-
Regulation of RNA methylation by modified histones.Nat Rev Genet. 2019 May;20(5):254-255. doi: 10.1038/s41576-019-0115-5. Nat Rev Genet. 2019. PMID: 30890788 No abstract available.
References
-
- Egger G, Liang G, Aparicio A & Jones PA Epigenetics in human disease and prospects for epigenetic therapy. Nature 429, 457–463 (2004) - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RM1 HG008935/HG/NHGRI NIH HHS/United States
- R01 CA236399/CA/NCI NIH HHS/United States
- R01 CA182528/CA/NCI NIH HHS/United States
- R01 CA214965/CA/NCI NIH HHS/United States
- R01 CA172558/CA/NCI NIH HHS/United States
- R56 DK120282/DK/NIDDK NIH HHS/United States
- R01 CA211066/CA/NCI NIH HHS/United States
- R01 CA243386/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 CA213138/CA/NCI NIH HHS/United States
- R01 CA157644/CA/NCI NIH HHS/United States
- R21 CA187276/CA/NCI NIH HHS/United States
- R01 CA178454/CA/NCI NIH HHS/United States
- R01 CA211614/CA/NCI NIH HHS/United States
- R35 CA197628/CA/NCI NIH HHS/United States
- R01 CA163493/CA/NCI NIH HHS/United States
- R01 CA139032/CA/NCI NIH HHS/United States
- P30 CA033572/CA/NCI NIH HHS/United States
- U10 CA180827/CA/NCI NIH HHS/United States
- R01 CA137060/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

