Histone serotonylation is a permissive modification that enhances TFIID binding to H3K4me3
- PMID: 30867594
- PMCID: PMC6557285
- DOI: 10.1038/s41586-019-1024-7
Histone serotonylation is a permissive modification that enhances TFIID binding to H3K4me3
Abstract
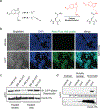
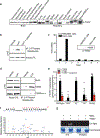
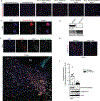
Chemical modifications of histones can mediate diverse DNA-templated processes, including gene transcription1-3. Here we provide evidence for a class of histone post-translational modification, serotonylation of glutamine, which occurs at position 5 (Q5ser) on histone H3 in organisms that produce serotonin (also known as 5-hydroxytryptamine (5-HT)). We demonstrate that tissue transglutaminase 2 can serotonylate histone H3 tri-methylated lysine 4 (H3K4me3)-marked nucleosomes, resulting in the presence of combinatorial H3K4me3Q5ser in vivo. H3K4me3Q5ser displays a ubiquitous pattern of tissue expression in mammals, with enrichment observed in brain and gut, two organ systems responsible for the bulk of 5-HT production. Genome-wide analyses of human serotonergic neurons, developing mouse brain and cultured serotonergic cells indicate that H3K4me3Q5ser nucleosomes are enriched in euchromatin, are sensitive to cellular differentiation and correlate with permissive gene expression, phenomena that are linked to the potentiation of TFIID4-6 interactions with H3K4me3. Cells that ectopically express a H3 mutant that cannot be serotonylated display significantly altered expression of H3K4me3Q5ser-target loci, which leads to deficits in differentiation. Taken together, these data identify a direct role for 5-HT, independent from its contributions to neurotransmission and cellular signalling, in the mediation of permissive gene expression.
Conflict of interest statement
Competing interest statement
The authors declare no competing financial interests.
Figures









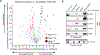
Comment in
-
Modification of histone proteins by serotonin in the nucleus.Nature. 2019 Mar;567(7749):464-465. doi: 10.1038/d41586-019-00532-z. Nature. 2019. PMID: 30911154 No abstract available.
-
Histone serotonylation boosts neuronal transcription.Nat Rev Mol Cell Biol. 2019 Jun;20(6):323. doi: 10.1038/s41580-019-0124-4. Nat Rev Mol Cell Biol. 2019. PMID: 30944456 No abstract available.
-
Histone Serotonylation: Can the Brain Have "Happy" Chromatin?Mol Cell. 2019 May 2;74(3):418-420. doi: 10.1016/j.molcel.2019.04.017. Mol Cell. 2019. PMID: 31051139 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH116900/MH/NIMH NIH HHS/United States
- P01 CA196539/CA/NCI NIH HHS/United States
- T32 DA007135/DA/NIDA NIH HHS/United States
- R01 CA204639/CA/NCI NIH HHS/United States
- DP1 DA042078/DA/NIDA NIH HHS/United States
- R01 CA129325/CA/NCI NIH HHS/United States
- R21 DA044767/DA/NIDA NIH HHS/United States
- T32 DA028874/DA/NIDA NIH HHS/United States
- R21 DA040837/DA/NIDA NIH HHS/United States
- R37 GM086868/GM/NIGMS NIH HHS/United States
- P50 MH096890/MH/NIMH NIH HHS/United States
- R01 GM110174/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

