Comparison of different cell culture plates for the enrichment of non-adherent human mononuclear cells
- PMID: 30867690
- PMCID: PMC6395970
- DOI: 10.3892/etm.2019.7204
Comparison of different cell culture plates for the enrichment of non-adherent human mononuclear cells
Abstract
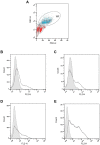
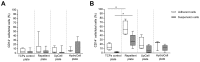
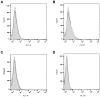
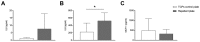
While tissue-resident monocytes and macrophages are considered to be vital players in the in vivo interaction between biomaterials and surrounding tissue, their isolation is limited. In order to establish in vitro models elucidating implant and tissue interactions, peripheral blood mononuclear cells (PBMCs) represent a viable source for bone marrow-derived monocytes and an alternative to tissue-resident cells. The aim of present study was to analyse different adhesion-preventing tissue culture plates for their potential to facilitate the culture of monocytes without differentiation into macrophages. Freshly isolated PBMCs were seeded into four commercially available tissue culture plates with different adhesive properties and were tested for surface CD14 and CD68 expression using flow cytometry following 7 days in culture. When PBMCs were cultivated in RPMI on Cellstar® Cell culture plates with Cell-Repellent Surface, a significant increase in CD14-positive cells was observed compared with cultivation in standard tissue culture-treated plates. This was accompanied by elevated cytokine production of interleukin-6 (IL6) and interleukin-8 (IL8); however, overall cell growth was not affected. When PBMCs were pre-cultured in cell-repellent plates, there was a higher yield of adherent cells after subsequent transfer into standard tissue culture-treated plates. Cultivation of PBMCs on cell-repellent culture plates favoured a monocytic phenotype and thus, represents an alternative to increase the fraction of monocytes yielded from PBMCs.
Keywords: adhesion; differentiation; hydrophilic; hydrophobic; monocytes; peripheral blood mononuclear cells.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
