Microporous polysaccharide multilayer coated BCP composite scaffolds with immobilised calcitriol promote osteoporotic bone regeneration both in vitro and in vivo
- PMID: 30867820
- PMCID: PMC6401415
- DOI: 10.7150/thno.29566
Microporous polysaccharide multilayer coated BCP composite scaffolds with immobilised calcitriol promote osteoporotic bone regeneration both in vitro and in vivo
Erratum in
-
Erratum: Microporous polysaccharide multilayer coated BCP composite scaffolds with immobilised calcitriol promote osteoporotic bone regeneration both in vitro and in vivo: Erratum.Theranostics. 2021 Apr 20;11(13):6524-6525. doi: 10.7150/thno.61641. eCollection 2021. Theranostics. 2021. PMID: 33995673 Free PMC article.
Abstract
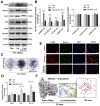
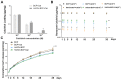
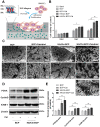
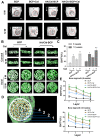
Incorporating a biomimetic coating and integrating osteoinductive biomolecules into basic bone substitutes are two common strategies to improve osteogenic capabilities in bone tissue engineering. Currently, the underlying mechanism of osteoporosis (OP)-related deficiency of osteogenesis remains unclear, and few treatments target at OP-related bone regeneration. Herein, we describe a self-assembling polyelectrolyte multilayered (PEM) film coating with local immobilisation of calcitriol (Cal) in biphasic calcium phosphate (BCP) scaffolds to promote osteoporotic bone regeneration by targeting the calcium sensing receptor (CaSR). Methods: The ovariectomy-induced functional changes in bone marrow mesenchymal stem cells (BMSCs), protective effects of Cal, and the potential mechanism were all verified. A PEM film composed of hyaluronic acid (HA) and chitosan (Chi) was prepared through layer-by-layer self-assembly. The morphology, growth behaviour, and drug retention capability of the composite scaffolds were characterised, and their biocompatibility and therapeutic efficacy for bone regeneration were systematically explored in vitro and in vivo.Results: The osteogenic differentiation, adhesion, and proliferation abilities of ovariectomised rat BMSCs (OVX-rBMSCs) decreased, in accordance with the deficiency of CaSR. Cal effectively activated osteogenesis in these OVX-rBMSCs by binding specifically to the active pocket of the CaSR structure, while the biomimetic PEM coating augmented OVX-rBMSCs proliferation and adhesion due to its porous surface structure. The PEM-coated scaffolds showed advantages in Cal loading and retention, especially at lower drug concentrations. HA/Chi PEM synergised with Cal to improve the proliferation, adhesion, and osteogenesis of OVX-rBMSCs and promote bone regeneration and BCP degradation in the critical-size calvarial bone defect model of OVX rats. Conclusion: A composite scaffold based on BCP, created by simply combining a biomimetic PEM coating and Cal immobilisation, could be clinically useful and has marked advantages as a targeted, off-the-shelf, cell-free treatment option for osteoporotic bone regeneration.
Keywords: calcitriol; critical-size bone defect; layer-by-layer assembly; osteoporosis; polysaccharide.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



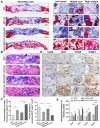
References
-
- Verron E, Gauthier O, Janvier P, Pilet P, Lesoeur J, Bujoli B. et al. In vivo bone augmentation in an osteoporotic environment using bisphosphonate-loaded calcium deficient apatite. Biomaterials. 2010;31:7776–84. - PubMed
-
- Cooper C, Campion G, Melton LJ 3rd. Hip fractures in the elderly: a world-wide projection. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 1992;2:285–9. - PubMed
-
- Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S. et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2014;29:2520–6. - PMC - PubMed
-
- Bonn D. New ways with old bones. Osteoporosis researchers look for drugs to replace hormone replacement therapy. Lancet. 2004;363:786–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

