Modular co-option of cardiopharyngeal genes during non-embryonic myogenesis
- PMID: 30867897
- PMCID: PMC6399929
- DOI: 10.1186/s13227-019-0116-7
Modular co-option of cardiopharyngeal genes during non-embryonic myogenesis
Abstract
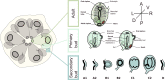
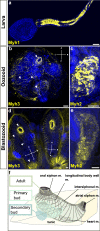
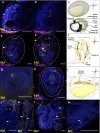
Background: In chordates, cardiac and body muscles arise from different embryonic origins. In addition, myogenesis can be triggered in adult organisms, during asexual development or regeneration. In non-vertebrate chordates like ascidians, muscles originate from embryonic precursors regulated by a conserved set of genes that orchestrate cell behavior and dynamics during development. In colonial ascidians, besides embryogenesis and metamorphosis, an adult can propagate asexually via blastogenesis, skipping embryo and larval stages, and form anew the adult body, including the complete body musculature.
Results: To investigate the cellular origin and mechanisms that trigger non-embryonic myogenesis, we followed the expression of ascidian myogenic genes during Botryllus schlosseri blastogenesis and reconstructed the dynamics of muscle precursors. Based on the expression dynamics of Tbx1/10, Ebf, Mrf, Myh3 for body wall and of FoxF, Tbx1/10, Nk4, Myh2 for heart development, we show that the embryonic factors regulating myogenesis are only partially co-opted in blastogenesis, and that markers for muscle precursors are expressed in two separate domains: the dorsal tube and the ventral mesenchyma.
Conclusions: Regardless of the developmental pathway, non-embryonic myogenesis shares a similar molecular and anatomical setup as embryonic myogenesis, but implements a co-option and loss of molecular modules. We then propose that the cellular precursors contributing to heart and body muscles may have different origins and may be coordinated by different developmental pathways.
Keywords: Ascidians; Blastogenesis; Botryllus schlosseri; Budding; Muscle; Regeneration.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

