The Y-linked proto-oncogene TSPY contributes to poor prognosis of the male hepatocellular carcinoma patients by promoting the pro-oncogenic and suppressing the anti-oncogenic gene expression
- PMID: 30867900
- PMCID: PMC6399826
- DOI: 10.1186/s13578-019-0287-x
The Y-linked proto-oncogene TSPY contributes to poor prognosis of the male hepatocellular carcinoma patients by promoting the pro-oncogenic and suppressing the anti-oncogenic gene expression
Abstract
Background: Liver cancer is one of the major causes of cancer death worldwide, with significantly higher incidence and mortality among the male patients. Although sex hormones and their receptors could contribute to such sex differences, the story is incomplete. Genes on the male-specific region of the Y chromosome could play a role(s) in this cancer. TSPY is the putative gene for the gonadoblastoma locus on the Y chromosome (GBY) that is ectopically expressed in a subset of male hepatocellular carcinomas (HCCs). Although various studies showed that TSPY expression is associated with poor prognosis in the patients and its overexpression promotes cell proliferation of various cancer cell lines, it remains unclear how TSPY contributes to the clinical outcomes of the HCC patients. Identifying the downstream genes and pathways of TSPY actions would provide novel insights on its contribution(s) to male predominance in this deadly cancer.
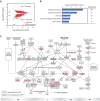
Results: To determine the effects of TSPY on HCC, a TSPY transgene was introduced to the HCC cell line, HuH-7, and studied with RNA-Seq transcriptome analysis. The results showed that TSPY upregulates various genes associated with cell-cycle and cell-viability, and suppresses cell-death related genes. To correlate the experimental observations with those of clinical specimens, transcriptomes of male HCCs with high TSPY expression were analyzed with reference to those with silent TSPY expression from the Cancer Genome Atlas (TCGA). The comparative analysis identified 49 genes, which showed parallel expression patterns between HuH-7 cells overexpressing TSPY and clinical specimens with high TSPY expression. Among these 49 genes, 16 likely downstream genes could be associated with survival rates in HCC patients. The major upregulated targets were cell-cycle related genes and growth factor receptor genes, including CDC25B and HMMR, whose expression levels are negatively correlated with the patient survival rates. In contrast, PPARGC1A, SLC25A25 and SOCS2 were downregulated with TSPY expression, and possess favorable prognoses for HCC patients.
Conclusion: We demonstrate that TSPY could exacerbate the oncogenesis of HCC by differentially upregulate the expression of pro-oncogenic genes and downregulate those of anti-oncogenic genes in male HCC patients, thereby contributing to the male predominance in this deadly cancer.
Keywords: Datamining; Hepatocellular carcinoma; Male predominance; TCGA dataset; TSPY; Transcriptome analysis; Y-chromosome.
Figures




References
-
- Salo P, Kaariainen H, Petrovic V, Peltomaki P, Page DC, de la Chapelle A. Molecular mapping of the putative gonadoblastoma locus on the Y chromosome. Genes Chromosom Cancer. 1995;14(3):210–214. - PubMed
-
- Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, Cordum HS, Hillier L, Brown LG, Repping S, Pyntikova T, Ali J, Bieri T, et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature. 2003;423(6942):825–837. - PubMed
-
- Schnieders F, Dork T, Arnemann J, Vogel T, Werner M, Schmidtke J. Testis-specific protein, Y-encoded (TSPY) expression in testicular tissues. Hum Mol Genet. 1996;5(11):1801–1807. - PubMed
-
- Zhang JS, Yang-Feng TL, Muller U, Mohandas TK, de Jong PJ, Lau YF. Molecular isolation and characterization of an expressed gene from the human Y chromosome. Hum Mol Genet. 1992;1(9):717–726. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

