In vitro observation: the GFP- E. coli adhering to porcine erythrocytes can be removed by porcine alveolar macrophages
- PMID: 30867982
- PMCID: PMC6410693
- DOI: 10.7717/peerj.6439
In vitro observation: the GFP- E. coli adhering to porcine erythrocytes can be removed by porcine alveolar macrophages
Abstract
Although the activation of pathogen phagocytosis via complement system has been studied, erythrocyte-phagocyte interactions in pigs are not clearly understood. Therefore, we sought to investigate the ability of porcine erythrocytes to clear immune complexes (ICs) by using laser confocal microscopy and flow cytometry to observe the immune adhesion of porcine erythrocytes to fluorescent bacilli and the immune presentation process of transferring fluorescent bacilli to macrophages. Isolated porcine alveolar macrophages (PAMs) had uniform morphology and size, and a survival rate of 97.2%. The phagocytosis rate was 98.8%. After WT E. coli was labeled with Fluorescein Isothiocyanate (FITC), the bacteria showed a bright green fluorescence, and the labeling rate was 92.3%. When laser confocal microscopy was utilized to observe the co-incubation system of porcine erythrocytes, PAM, and fluorescent E. coli, the fluorescence intensity of bacilli decreased with increasing observation time and even disappeared. Flow Cytometry examination showed that the average fluorescence intensity of PAMs co-incubated with porcine erythrocytes adhered to WT-E. coli-FITC, was significantly higher than that of normal PAMs. Furthermore, when porcine erythrocytes adhered to WT E. coli were incubated with PAMs, the surface mean fluorescence intensity of porcine erythrocytes was significantly higher than that of the blank control group. This shows that PAMs can competitively bind to the oposinized E. coli adhered to the surface of porcine erythrocytes, and these oposinized pathogens can enter macrophages by the process of phagocytosis, which promoting the internalization of ICs or pathogens. During this process, the physical morphology of porcine erythrocytes was not damaged, but the levels of its main functional protein CR1-like were reduced.
Keywords: CR1-like; GFP-E. coli removed; Porcine alveolar macrophages; Porcine erythrocytes.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



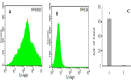
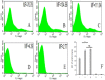
References
-
- BAO ENDON SU JIANDONG Studies on the red blood cell (RBC) immune function of periphery blood of chickens which embryonally vaccinated with marek’s disease vaccine. Acta Veterinaria et Zootechnica Sinica. 2000;31(5):436–440.
-
- Das N, Biswas B, Khera R. Membrane-bound complement regulatory proteins as biomarkers and potential therapeutic targets for SLE. Indian Journal of Experimental Biology. 2013;735:55–81. - PubMed
-
- Feng S, Cai X. Immunological research status of cysticercosis. Chinese Journal of Veterinary Science and Technology. 2000;30(8):15–18.
LinkOut - more resources
Full Text Sources
Miscellaneous

