Osteogenic capacity and cytotherapeutic potential of periodontal ligament cells for periodontal regeneration in vitro and in vivo
- PMID: 30867997
- PMCID: PMC6410690
- DOI: 10.7717/peerj.6589
Osteogenic capacity and cytotherapeutic potential of periodontal ligament cells for periodontal regeneration in vitro and in vivo
Abstract
Background: The periodontal ligament cells (PDLCs) contain heterogeneous cell populations and possess stem-cell-like properties. PDLCs have attracted considerable attention as an option for periodontal regeneration. However, the osteogenic differentiation of PDLCs remains obscure owing to variable osteo-inductive methods and whether PDLCs could be directly used for periodontal regeneration without stem cell enrichment is uncertain. The aim of the present study was to clarify the osteogenic differentiation capacity of PDLCs and test PDLCs as an alternative to stem cells for periodontal regeneration.
Methods: We tested the performance of human PDLCs in osteo-inductive culture and transplantation in vivo while taking human bone marrow derived mesenchymal stem cells (hMSCs) as positive control. Proliferation of PDLCs and hMSCs in osteo-inductive condition were examined by MTT assay and colony formation assay. The osteogenic differentiations of PDLCs and hMSCs were assessed by Alkaline phosphatase (ALP) activity measurement, von Kossa staining, Alizarin red S staining and quantitative RT-PCR of osteogenic marker gene including RUNX2, ALP, OCN, Col I, BSP, OPN. We transplanted osteo-inductive PDLCs and hMSCs with hydroxyapatite/tricalcium phosphate (HA/TCP) scaffolds to immunodeficient mice to explore their biological behaviors in vivo by histological staining and immunohistochemical evaluation.
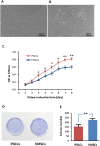
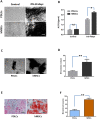
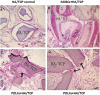
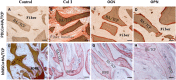
Results: After 14 days of osteo-induction, PDLCs exhibited significantly higher proliferation rate but lower colony-forming ability comparing with hMSCs. PDLCs demonstrated lower ALP activity and generated fewer mineralized nodules than hMSCs. PDLCs showed overall up-regulated expression of RUNX2, ALP, OCN, Col I, BSP, OPN after osteo-induction. Col I level of PDLCs in osteo-inductive group was significantly higher while RUNX2, ALP, OCN were lower than that of hMSCs. Massive fiber bundles were produced linking or circling the scaffold while the bone-like structures were limited in the PDLCs-loaded HA/TCP samples. The fiber bundles displayed strong positive Col I, but weak OCN and OPN staining. The in vivo results were consistent with the in vitro data, which confirmed strong collagen forming ability and considerable osteogenic potential of PDLCs.
Conclusion: It is encouraging to find that PDLCs exhibit higher proliferation, stronger collagen fiber formation capacity, but lower osteogenic differentiation ability in comparison with hMSCs. This characteristic is essential for the successful periodontal reconstruction which is based on the synchronization of fiber formation and bone deposition. Moreover, PDLCs have advantages such as good accessibility, abundant source, vigorous proliferation and evident osteogenic differentiation capacity when triggered properly. They can independently form PDL-like structure in vivo without specific stem cell enrichment procedure. The application of PDLCs may offer a novel cytotherapeutic option for future clinical periodontal reconstruction.
Keywords: Human bone marrow derived mesenchymal stem cells (hMSCs); Human periodontal ligament cells (PDLCs); Osteogenic differentiation; Periodontal regeneration.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Aimetti M, Ferrarotti F, Mariani G, Fratini A, Giraudi M, Romano F. Enamel matrix derivative proteins in combination with a flapless approach for periodontal regeneration of intrabony defects: a 2-year prospective case series. International Journal of Periodontics & Restorative Dentistry. 2016;36(6):797–805. doi: 10.11607/prd.2842. - DOI - PubMed
-
- Akizuki T, Oda S, Komaki M, Tsuchioka H, Kawakatsu N, Kikuchi A, Yamato M, Okano T, Ishikawa I. Application of periodontal ligament cell sheet for periodontal regeneration: a pilot study in beagle dogs. Journal of Periodontal Research. 2005;40(3):245–251. doi: 10.1111/j.1600-0765.2005.00799.x. - DOI - PubMed
-
- An S, Gao Y, Huang X, Ling J, Liu Z, Xiao Y. A comparative study of the proliferation and osteogenic differentiation of human periodontal ligament cells cultured on β-TCP ceramics and demineralized bone matrix with or without osteogenic inducers in vitro. International Journal of Molecular Medicine. 2015;35(5):1341–1346. doi: 10.3892/ijmm.2015.2122. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

