Perspective: How can ultrafast laser spectroscopy inform the design of new organic photoredox catalysts for chemical and materials synthesis?
- PMID: 30868082
- PMCID: PMC6404927
- DOI: 10.1063/1.5082620
Perspective: How can ultrafast laser spectroscopy inform the design of new organic photoredox catalysts for chemical and materials synthesis?
Abstract
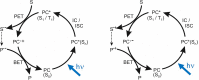
Photoredox catalysis of chemical reactions, using light-activated molecules which serve as electron donors or acceptors to initiate chemical transformations under mild conditions, is finding widespread use in the synthesis of organic compounds and materials. The transition-metal-centred complexes first developed for these photoredox-catalysed applications are steadily being superseded by more sustainable and lower toxicity organic photocatalysts. While the diversity of possible structures for photoredox-active organic molecules brings benefits of design flexibility, it also presents considerable challenges for optimization of the photocatalyst molecular architecture. Transient absorption spectroscopy over timescales from the femtosecond to microsecond domains can explore the detailed mechanisms of activation and reaction of these organic photocatalysts in solution and, by linking their dynamical properties to their structures, has the potential to establish reliable design principles for future development of improved photocatalysts.
Figures


References
-
- Twilton J., Le C., Zhang P., Shaw M. H., Evans R. W., and MacMillan D. W. C., Nat. Rev. Chem. 1, 0052 (2017).10.1038/s41570-017-0052 - DOI
LinkOut - more resources
Full Text Sources

