Targeting the Small GTPase Superfamily through Their Regulatory Proteins
- PMID: 30869179
- PMCID: PMC7204875
- DOI: 10.1002/anie.201900585
Targeting the Small GTPase Superfamily through Their Regulatory Proteins
Abstract
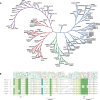
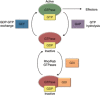
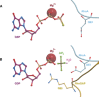
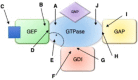
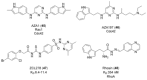
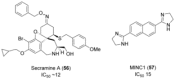
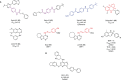
The Ras superfamily of small GTPases are guanine-nucleotide-dependent switches essential for numerous cellular processes. Mutations or dysregulation of these proteins are associated with many diseases, but unsuccessful attempts to target the small GTPases directly have resulted in them being classed as "undruggable". The GTP-dependent signaling of these proteins is controlled by their regulators; guanine nucleotide exchange factors (GEFs), GTPase activating proteins (GAPs), and in the Rho and Rab subfamilies, guanine nucleotide dissociation inhibitors (GDIs). This review covers the recent small molecule and biologics strategies to target the small GTPases through their regulators. It seeks to critically re-evaluate recent chemical biology practice, such as the presence of PAINs motifs and the cell-based readout using compounds that are weakly potent or of unknown specificity. It highlights the vast scope of potential approaches for targeting the small GTPases in the future through their regulatory proteins.
Keywords: drug discovery; peptides; protein-protein interactions; small GTPases; small molecules.
© 2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






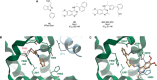







References
-
- Wennerberg K., Rossman K. L., Der C. J., J. Cell Sci. 2005, 118, 843–846. - PubMed
-
- None
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

