Prophylactic Cranial Irradiation vs Observation in Patients With Locally Advanced Non-Small Cell Lung Cancer: A Long-term Update of the NRG Oncology/RTOG 0214 Phase 3 Randomized Clinical Trial
- PMID: 30869743
- PMCID: PMC6459052
- DOI: 10.1001/jamaoncol.2018.7220
Prophylactic Cranial Irradiation vs Observation in Patients With Locally Advanced Non-Small Cell Lung Cancer: A Long-term Update of the NRG Oncology/RTOG 0214 Phase 3 Randomized Clinical Trial
Abstract
Importance: Brain metastasis (BM) rates are high in locally advanced non-small cell lung cancer (LA-NSCLC), approaching rates seen in small cell lung cancer, where prophylactic cranial irradiation (PCI) is standard of care. Although PCI decreases the incidence of BM in LA-NSCLC, a survival advantage has not yet been shown.
Objective: To determine if PCI improves survival in LA-NSCLC.
Design, setting, and participants: Radiation Therapy Oncology Group (RTOG) 0214 was a randomized phase 3 clinical trial in stage III NSCLC stratified by stage (IIIA vs IIIB), histologic characteristics (nonsquamous vs squamous) and therapy (no surgery vs surgery). The study took place at 291 institutions in the United States, Canada, and internationally. Of 356 patients with stage III NSCLC entered onto this study, 16 were ineligible; therefore, 340 patients were randomized.
Intervention for clinical trials: Observation vs PCI.
Main outcomes and measures: The primary outcome was overall survival (OS). The secondary end points were disease-free survival (DFS) and incidence of BM.
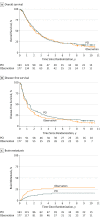
Results: Of the 340 total participants, mean (SD) age was 61 years; 213 of the participants were men and 127 were women. The median follow-up time was 2.1 years for all patients, and 9.2 years for living patients. The OS for PCI was not significantly better than observation (hazard ratio [HR], 0.82; 95% CI, 0.63-1.06; P = .12; 5- and 10-year rates, 24.7% and 17.6% vs 26.0% and 13.3%, respectively), while the DFS (HR, 0.76; 95% CI, 0.59-0.97; P = .03; 5- and 10-year rates, 19.0% and 12.6% vs 16.1% and 7.5% for PCI vs observation) and BM (HR, 0.43; 95% CI, 0.24-0.77; P = .003; 5- and 10-year rates, 16.7% vs 28.3% for PCI vs observation) were significantly different. Patients in the PCI arm were 57% less likely to develop BM than those in the observation arm. Younger patients (<60 years) and patients with nonsquamous disease developed more BM. On multivariable analysis, PCI was associated with decreased BM and improved DFS, but not improved OS. Multivariable analysis within the nonsurgical arm suggests that PCI effectively prolongs OS, DFS, and BM.
Conclusions and relevance: In patients with stage III LA-NSCLC without progression of disease after therapy, PCI decreased the 5- and 10-year rate of BM and improved 5- and 10-year DFS, but did not improve OS. Although this study did not meet its primary end point, the long-term results reveal many important findings that will benefit future trials. Identifying the appropriate patient population and a safe intervention is critical.
Trial registration: ClinicalTrials.gov identifier: NCT00048997.
Conflict of interest statement
Figures
References
-
- Chen AM, Jahan TM, Jablons DM, Garcia J, Larson DA. Risk of cerebral metastases and neurological death after pathological complete response to neoadjuvant therapy for locally advanced nonsmall-cell lung cancer: clinical implications for the subsequent management of the brain. Cancer. 2007;109(8):1668-1675. doi: 10.1002/cncr.22565 - DOI - PubMed
-
- Pöttgen C, Eberhardt W, Grannass A, et al. Prophylactic cranial irradiation in operable stage IIIA non small-cell lung cancer treated with neoadjuvant chemoradiotherapy: results from a German multicenter randomized trial. J Clin Oncol. 2007;25(31):4987-4992. doi: 10.1200/JCO.2007.12.5468 - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous