Endometrial cells contribute to preexisting endometriosis lesions in a mouse model of retrograde menstruation†
- PMID: 30869747
- PMCID: PMC6561859
- DOI: 10.1093/biolre/ioz039
Endometrial cells contribute to preexisting endometriosis lesions in a mouse model of retrograde menstruation†
Abstract
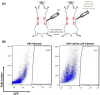
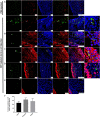
Endometriosis is characterized by extrauterine growth of endometrial tissue accompanied by adverse clinical manifestations including chronic pelvic pain and infertility. Retrograde menstruation, the efflux of endometrium into the peritoneal cavity during menstruation, is believed to contribute to implantation of endometrial tissue and formation of endometriotic lesions at ectopic sites. While it is established through various rodent and nonhuman primate models that endometrial tissue fragments, as well as nondissociated stroma and glands, are capable of seeding endometriosis in a manner mimicking retrograde menstruation, the ability of single endometrial cells to participate in endometriotic processes has not been evaluated due to their failure to establish macroscopic endometriosis. We designed a model by which this capacity can be assessed by examining the integration of individual uterine cells into existing endometriosis lesions in mice. Endometriosis was induced in C57BL/6J female mice followed by intraperitoneal injection of GFP-labeled single uterine cells. We found that freshly introduced uterine cells can successfully integrate and contribute to various cell populations within the lesion. Strikingly, these cells also appeared to contribute to neo-angiogenesis and inflammatory processes within the lesion, which are commonly thought of as host-driven phenomena. Our findings underscore the potential of individual uterine cells to continuously expand lesions and participate in the progression of endometriosis. This model of retrograde menstruation may therefore be used to study processes involved in the pathophysiology of endometriosis.
Keywords: endometriosis; endometrium; retrograde menstruation.
© The Author(s) 2019. Published by Oxford University Press on behalf of Society for the Study of Reproduction.
Figures


References
-
- Simoens S, Hummelshoj L, D’Hooghe T. Endometriosis: cost estimates and methodological perspective. Hum Reprod Update 2007; 13(4):395–404. - PubMed
-
- Culley L, Law C, Hudson N, Denny E, Mitchell H, Baumgarten M, Raine-Fenning N. The social and psychological impact of endometriosis on women's lives: a critical narrative review. Hum Reprod Update 2013; 19(6):625–639. - PubMed
-
- Gordts S, Koninckx P, Brosens I. Pathogenesis of deep endometriosis. Fertil Steril 2017; 108(6):872–885.e1. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

