Establishing the Link Between Lean Mass and Grip Strength Cut Points With Mobility Disability and Other Health Outcomes: Proceedings of the Sarcopenia Definition and Outcomes Consortium Conference
- PMID: 30869772
- PMCID: PMC7447857
- DOI: 10.1093/gerona/glz081
Establishing the Link Between Lean Mass and Grip Strength Cut Points With Mobility Disability and Other Health Outcomes: Proceedings of the Sarcopenia Definition and Outcomes Consortium Conference
Abstract
Background: Lack of consensus on how to diagnose sarcopenia has limited the ability to diagnose this condition and hindered drug development. The Sarcopenia Definitions and Outcomes Consortium (SDOC) was formed to develop evidence-based diagnostic cut points for lean mass and/or muscle strength that identify people at increased risk of mobility disability. We describe here the proceedings of a meeting of SDOC and other experts to discuss strategic considerations in the development of evidence-based sarcopenia definition.
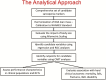
Methods: Presentations and panel discussions reviewed the usefulness of sarcopenia as a biomarker, the analytical approach used by SDOC to establish cut points, and preliminary findings, and provided strategic direction to develop an evidence-based definition of sarcopenia.
Results: The SDOC assembled data from eight epidemiological cohorts consisting of 18,831 participants, clinical populations from 10 randomized trials and observational studies, and 2 nationally representative cohorts. In preliminary assessments, grip strength or grip strength divided by body mass index was identified as discriminators of risk for mobility disability (walking speed <0.8 m/s), whereas dual-energy X-ray absorptiometry-derived lean mass measures were not good discriminators of mobility disability. Candidate definitions based on grip strength variables were associated with increased risk of mortality, falls, mobility disability, and instrumental activities of daily living disability. The prevalence of low grip strength increased with age. The attendees recommended the establishment of an International Expert Panel to review a series of position statements on sarcopenia definition that are informed by the findings of the SDOC analyses and synthesis of literature.
Conclusions: International consensus on an evidence-based definition of sarcopenia is needed. Grip strength-absolute or adjusted for body mass index-is an important discriminator of mobility disability and other endpoints. Additional research is needed to develop a predictive risk model that takes into account sarcopenia components as well as age, sex, race, and comorbidities.
Keywords: Grip strength cut-points; Lean mass cut-points; Mobility disability; Risk factors for mobility disability; Sarcopenia.
© The Author(s) 2019. Published by Oxford University Press on behalf of The Gerontological Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Evans WJ, Campbell WW. Sarcopenia and age-related changes in body composition and functional capacity. J Nutr. 1993;123(2 Suppl):465–468. doi: 10.1093/jn/123.suppl_2.465 - PubMed
-
- Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. - PubMed
Publication types
MeSH terms
Grants and funding
- U01 AI035042/AI/NIAID NIH HHS/United States
- U01 AG042145/AG/NIA NIH HHS/United States
- U01 AG042140/AG/NIA NIH HHS/United States
- U01 AG042124/AG/NIA NIH HHS/United States
- HHSN268201200036C/HL/NHLBI NIH HHS/United States
- N01 AG062106/AG/NIA NIH HHS/United States
- N01 HC035129/HC/NHLBI NIH HHS/United States
- U01 AG042139/AG/NIA NIH HHS/United States
- N01 HC045133/HC/NHLBI NIH HHS/United States
- R01 AG005407/AG/NIA NIH HHS/United States
- U01 AG042168/AG/NIA NIH HHS/United States
- R01 AG027574/AG/NIA NIH HHS/United States
- U01 AG051421/AG/NIA NIH HHS/United States
- N01HC85080/HL/NHLBI NIH HHS/United States
- P30 AG024827/AG/NIA NIH HHS/United States
- N01 AG062103/AG/NIA NIH HHS/United States
- R01 AR035583/AR/NIAMS NIH HHS/United States
- P30 AG028747/AG/NIA NIH HHS/United States
- N01 HC085084/HC/NHLBI NIH HHS/United States
- R37 AG009901/AG/NIA NIH HHS/United States
- UL1 TR000128/TR/NCATS NIH HHS/United States
- U24 AG051129/AG/NIA NIH HHS/United States
- R01 AG027576/AG/NIA NIH HHS/United States
- N01HC85082/HL/NHLBI NIH HHS/United States
- R01 AR041398/AR/NIAMS NIH HHS/United States
- U01 AG042143/AG/NIA NIH HHS/United States
- P30 AG028740/AG/NIA NIH HHS/United States
- R01 AR035584/AR/NIAMS NIH HHS/United States
- N01HC55222/HL/NHLBI NIH HHS/United States
- R01 AR057118/AR/NIAMS NIH HHS/United States
- N01HC85079/HL/NHLBI NIH HHS/United States
- N01HC85086/HL/NHLBI NIH HHS/United States
- N01 HC015103/HC/NHLBI NIH HHS/United States
- N01 AG062101/AG/NIA NIH HHS/United States
- U01 HL146201/HL/NHLBI NIH HHS/United States
- N01HC75150/HL/NHLBI NIH HHS/United States
- N01 HC025195/HC/NHLBI NIH HHS/United States
- N01 HC085085/HC/NHLBI NIH HHS/United States
- U01 AG027810/AG/NIA NIH HHS/United States
- R01 AR035582/AR/NIAMS NIH HHS/United States
- U01 AR066160/AR/NIAMS NIH HHS/United States
- P60 AR049465/AR/NIAMS NIH HHS/United States
- R01 AG005394/AG/NIA NIH HHS/United States
- R01 AR049439/AR/NIAMS NIH HHS/United States
- N01HC85081/HL/NHLBI NIH HHS/United States