Inorganic nitrate, hypoxia, and the regulation of cardiac mitochondrial respiration-probing the role of PPARα
- PMID: 30870003
- PMCID: PMC6529343
- DOI: 10.1096/fj.201900067R
Inorganic nitrate, hypoxia, and the regulation of cardiac mitochondrial respiration-probing the role of PPARα
Abstract
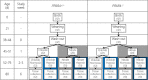
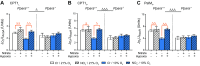
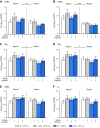
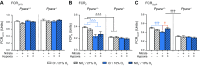
Dietary inorganic nitrate prevents aspects of cardiac mitochondrial dysfunction induced by hypoxia, although the mechanism is not completely understood. In both heart and skeletal muscle, nitrate increases fatty acid oxidation capacity, and in the latter case, this involves up-regulation of peroxisome proliferator-activated receptor (PPAR)α expression. Here, we investigated whether dietary nitrate modifies mitochondrial function in the hypoxic heart in a PPARα-dependent manner. Wild-type (WT) mice and mice without PPARα (Ppara-/-) were given water containing 0.7 mM NaCl (control) or 0.7 mM NaNO3 for 35 d. After 7 d, mice were exposed to normoxia or hypoxia (10% O2) for the remainder of the study. Mitochondrial respiratory function and metabolism were assessed in saponin-permeabilized cardiac muscle fibers. Environmental hypoxia suppressed mass-specific mitochondrial respiration and additionally lowered the proportion of respiration supported by fatty acid oxidation by 18% (P < 0.001). This switch away from fatty acid oxidation was reversed by nitrate treatment in hypoxic WT but not Ppara-/- mice, indicating a PPARα-dependent effect. Hypoxia increased hexokinase activity by 33% in all mice, whereas lactate dehydrogenase activity increased by 71% in hypoxic WT but not Ppara-/- mice. Our findings indicate that PPARα plays a key role in mediating cardiac metabolic remodeling in response to both hypoxia and dietary nitrate supplementation.-Horscroft, J. A., O'Brien, K. A., Clark, A. D., Lindsay, R. T., Steel, A. S., Procter, N. E. K., Devaux, J., Frenneaux, M., Harridge, S. D. R., Murray, A. J. Inorganic nitrate, hypoxia, and the regulation of cardiac mitochondrial respiration-probing the role of PPARα.
Keywords: fatty acids; heart; metabolism; mitochondria.
Conflict of interest statement
The authors acknowledge the support of Prof. Kieran Clarke (University of Oxford, Oxford, United Kingdom) and Prof. Martin Feelisch (University of Southampton, Southampton, United Kingdom). This work was supported by the Biotechnology and Biological Sciences Research Council, UK (Grant BB/F016581/1) and the Research Councils UK (Grant EP/E500552/1). The authors declare no conflicts of interest.
Figures



Similar articles
-
PPARα-independent effects of nitrate supplementation on skeletal muscle metabolism in hypoxia.Biochim Biophys Acta Mol Basis Dis. 2019 Apr 1;1865(4):844-853. doi: 10.1016/j.bbadis.2018.07.027. Epub 2018 Jul 25. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 30055294 Free PMC article.
-
PPARα augments heart function and cardiac fatty acid oxidation in early experimental polymicrobial sepsis.Am J Physiol Heart Circ Physiol. 2017 Feb 1;312(2):H239-H249. doi: 10.1152/ajpheart.00457.2016. Epub 2016 Nov 23. Am J Physiol Heart Circ Physiol. 2017. PMID: 27881386 Free PMC article.
-
On the pivotal role of PPARα in adaptation of the heart to hypoxia and why fat in the diet increases hypoxic injury.FASEB J. 2016 Aug;30(8):2684-97. doi: 10.1096/fj.201500094R. Epub 2016 Apr 21. FASEB J. 2016. PMID: 27103577 Free PMC article.
-
The role and regulation of the peroxisome proliferator activated receptor alpha in human liver.Biochimie. 2017 May;136:75-84. doi: 10.1016/j.biochi.2016.12.019. Epub 2017 Jan 8. Biochimie. 2017. PMID: 28077274 Review.
-
PPAR signaling in the control of cardiac energy metabolism.Trends Cardiovasc Med. 2000 Aug;10(6):238-45. doi: 10.1016/s1050-1738(00)00077-3. Trends Cardiovasc Med. 2000. PMID: 11282301 Review.
Cited by
-
Hypoxia-induced signaling in the cardiovascular system: pathogenesis and therapeutic targets.Signal Transduct Target Ther. 2023 Nov 20;8(1):431. doi: 10.1038/s41392-023-01652-9. Signal Transduct Target Ther. 2023. PMID: 37981648 Free PMC article. Review.
-
An optimized protocol for coupling oxygen consumption rates with β-oxidation in isolated mitochondria from mouse soleus.STAR Protoc. 2021 Aug 12;2(3):100735. doi: 10.1016/j.xpro.2021.100735. eCollection 2021 Sep 17. STAR Protoc. 2021. PMID: 34430910 Free PMC article.
-
Placental mitochondria in high-altitude pregnancy: metabolic adaptations and a toolkit for respiratory assessment.Philos Trans R Soc Lond B Biol Sci. 2025 Aug 21;380(1933):20240175. doi: 10.1098/rstb.2024.0175. Epub 2025 Aug 21. Philos Trans R Soc Lond B Biol Sci. 2025. PMID: 40836806 Free PMC article. Review.
-
The Glitazars Paradox: Cardiotoxicity of the Metabolically Beneficial Dual PPARα and PPARγ Activation.J Cardiovasc Pharmacol. 2020 Nov;76(5):514-526. doi: 10.1097/FJC.0000000000000891. J Cardiovasc Pharmacol. 2020. PMID: 33165133 Free PMC article. Review.
-
Beta-adrenergic agonism protects mitochondrial metabolism in the pancreatectomised rat heart.Sci Rep. 2024 Aug 21;14(1):19383. doi: 10.1038/s41598-024-70335-4. Sci Rep. 2024. PMID: 39169098 Free PMC article.
References
-
- Lopaschuk G. D., Ussher J. R., Folmes C. D., Jaswal J. S., Stanley W. C. (2010) Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 90, 207–258 - PubMed
-
- Belanger A. J., Luo Z., Vincent K. A., Akita G. Y., Cheng S. H., Gregory R. J., Jiang C. (2007) Hypoxia-inducible factor 1 mediates hypoxia-induced cardiomyocyte lipid accumulation by reducing the DNA binding activity of peroxisome proliferator-activated receptor alpha/retinoid X receptor. Biochem. Biophys. Res. Commun. 364, 567–572 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

