Serological proteomic screening and evaluation of a recombinant egg antigen for the diagnosis of low-intensity Schistosoma mansoni infections in endemic area in Brazil
- PMID: 30870412
- PMCID: PMC6472831
- DOI: 10.1371/journal.pntd.0006974
Serological proteomic screening and evaluation of a recombinant egg antigen for the diagnosis of low-intensity Schistosoma mansoni infections in endemic area in Brazil
Abstract
Background: Despite decades of use of control programs, schistosomiasis remains a global public health problem. To further reduce prevalence and intensity of infection, or to achieve the goal of elimination in low-endemic areas, there needs to be better diagnostic tools to detect low-intensity infections in low-endemic areas in Brazil. The rationale for development of new diagnostic tools is that the current standard test Kato-Katz (KK) is not sensitive enough to detect low-intensity infections in low-endemic areas. In order to develop new diagnostic tools, we employed a proteomics approach to identify biomarkers associated with schistosome-specific immune responses in hopes of developing sensitive and specific new methods for immunodiagnosis.
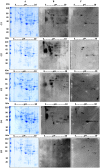
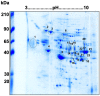
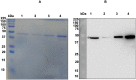
Methods and findings: Immunoproteomic analyses were performed on egg extracts of Schistosoma mansoni using pooled sera from infected or non-infected individuals from a low-endemic area of Brazil. Cross reactivity with other soil-transmitted helminths (STH) was determined using pooled sera from individuals uniquely infected with different helminths. Using this approach, we identified 23 targets recognized by schistosome acute and chronic sera samples. To identify immunoreactive targets that were likely glycan epitopes, we compared these targets to the immunoreactivity of spots treated with sodium metaperiodate oxidation of egg extract. This treatment yielded 12/23 spots maintaining immunoreactivity, suggesting that they were protein epitopes. From these 12 spots, 11 spots cross-reacted with sera from individuals infected with other STH and 10 spots cross-reacted with the negative control group. Spot number 5 was exclusively immunoreactive with sera from S. mansoni-infected groups in native and deglycosylated conditions and corresponds to Major Egg Antigen (MEA). We expressed MEA as a recombinant protein and showed a similar recognition pattern to that of the native protein via western blot. IgG-ELISA gave a sensitivity of 87.10% and specificity of 89.09% represented by area under the ROC curve of 0.95. IgG-ELISA performed better than the conventional KK (2 slides), identifying 56/64 cases harboring 1-10 eggs per gram of feces that were undiagnosed by KK parasitological technique.
Conclusions: The serological proteome approach was able to identify a new diagnostic candidate. The recombinant egg antigen provided good performance in IgG-ELISA to detect individuals with extreme low-intensity infections (1 egg per gram of feces). Therefore, the IgG-ELISA using this newly identified recombinant MEA can be a useful tool combined with other techniques in low-endemic areas to determine the true prevalence of schistosome infection that is underestimated by the KK method. Further, to overcome the complexity of ELISA in the field, a second generation of antibody-based rapid diagnostic tests (RDT) can be developed.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Evaluation of the CCA Immuno-Chromatographic Test to Diagnose Schistosoma mansoni in Minas Gerais State, Brazil.PLoS Negl Trop Dis. 2016 Jan 11;10(1):e0004357. doi: 10.1371/journal.pntd.0004357. eCollection 2016 Jan. PLoS Negl Trop Dis. 2016. PMID: 26752073 Free PMC article.
-
Evaluating a point-of-care circulating cathodic antigen test (POC-CCA) to detect Schistosoma mansoni infections in a low endemic area in north-eastern Brazil.Acta Trop. 2018 Jun;182:264-270. doi: 10.1016/j.actatropica.2018.03.002. Epub 2018 Mar 8. Acta Trop. 2018. PMID: 29526480
-
Evaluation of isotype-based serology for diagnosis of Schistosoma mansoni infection in individuals living in endemic areas with low parasite burden.Acta Trop. 2023 Dec;248:107017. doi: 10.1016/j.actatropica.2023.107017. Epub 2023 Sep 28. Acta Trop. 2023. PMID: 37774894
-
New approaches for the control and eradication of schistosomiasis in Venezuela.Mem Inst Oswaldo Cruz. 1992;87 Suppl 4:227-31. doi: 10.1590/s0074-02761992000800035. Mem Inst Oswaldo Cruz. 1992. PMID: 1343900 Review.
-
Evaluation, Validation, and Recognition of the Point-of-Care Circulating Cathodic Antigen, Urine-Based Assay for Mapping Schistosoma mansoni Infections.Am J Trop Med Hyg. 2020 Jul;103(1_Suppl):42-49. doi: 10.4269/ajtmh.19-0788. Am J Trop Med Hyg. 2020. PMID: 32400347 Free PMC article.
Cited by
-
Immunoblotting Identification of Diagnostic Antigens of Paragonimus westermani Type 1 for the Detection of Human Pulmonary Paragonimiasis in North East India.Trop Med Infect Dis. 2023 Dec 22;9(1):6. doi: 10.3390/tropicalmed9010006. Trop Med Infect Dis. 2023. PMID: 38251203 Free PMC article.
-
Evaluation of Synthetic Peptides from Schistosoma mansoni ATP Diphosphohydrolase 1: In Silico Approaches for Characterization and Prospective Application in Diagnosis of Schistosomiasis.ACS Infect Dis. 2025 Feb 14;11(2):463-473. doi: 10.1021/acsinfecdis.4c00697. Epub 2025 Jan 14. ACS Infect Dis. 2025. PMID: 39807991 Free PMC article.
-
Diagnostic performances of Schistosoma haematobium and Schistosoma mansoni recombinant proteins, peptides and chimeric proteins antibody based tests. Systematic scoping review.PLoS One. 2023 Mar 2;18(3):e0282233. doi: 10.1371/journal.pone.0282233. eCollection 2023. PLoS One. 2023. PMID: 36862712 Free PMC article.
-
Evaluation of schistosomula crude antigen (SCA) as a diagnostic tool for Schistosoma mansoni in low endemic human population.Sci Rep. 2021 May 18;11(1):10530. doi: 10.1038/s41598-021-89929-3. Sci Rep. 2021. PMID: 34006964 Free PMC article.
-
Evaluation of copromicroscopy and serology to measure the exposure to Ascaris infections across age groups and to assess the impact of 3 years of biannual mass drug administration in Jimma Town, Ethiopia.PLoS Negl Trop Dis. 2020 Apr 13;14(4):e0008037. doi: 10.1371/journal.pntd.0008037. eCollection 2020 Apr. PLoS Negl Trop Dis. 2020. PMID: 32282815 Free PMC article.
References
-
- WHO. Accelerating work to overcome the global impact of neglected tropical diseases A roadmap for implementation. 2012. [cited 2017 11/24]. http://www.who.int/neglected_diseases/NTD_RoadMap_2012_Fullversion.pdf?ua=1.
-
- WHO. Schistosomiasis: number of people treated worldwide in 2014. Wkly Epidemiol Rec. 2016;91(5):53–60. . - PubMed
-
- WHO/PAHO. Neglected infectious diseases in the Americas: Success stories and innovation to reach the neediest. 2016. [cited 2017 11/24]. http://www.paho.org/neglected-infectious-diseases-stories http://iris.paho.org/xmlui/handle/123456789/31250.
-
- Katz N. Inquérito Nacional de Prevalência da Esquistossomose mansoni e Geo-helmintoses (2010–2015). Belo Horizonte: Instituto Rene Rachou—Fiocruz, 2018 March, 2018. Report No.: Contract No.: k197.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

