In vitro model of postoncosphere development, and in vivo infection abilities of Taenia solium and Taenia saginata
- PMID: 30870421
- PMCID: PMC6435196
- DOI: 10.1371/journal.pntd.0007261
In vitro model of postoncosphere development, and in vivo infection abilities of Taenia solium and Taenia saginata
Abstract
Taenia solium is known to cause human cysticercosis while T. saginata does not. Comparative in vitro and in vivo studies on the oncosphere and the postoncospheral (PO) forms of T. solium and T. saginata may help to elucidate why cysticercosis can occur from one and not the other. The aim of this study was to use in vitro culture assays and in vivo models to study the differences in the development of the T. solium and T. saginata oncosphere. Furthermore, this study aimed to evaluate the expression of cytokines and metalloproteinases (MMPs) in human peripheral blood mononuclear cells (PBMCs), which were stimulated by these oncospheres and PO antigens. T. solium and T. saginata activated oncospheres (AO) were cultured in INT-407 and HCT-8 intestinal cells for 180 days. The T. solium began to die while the T. saginata grew for 180 days and developed to cysticerci in INT-407 cells. Rats were inoculated intracranially with AO and PO forms of either T. saginata or T. solium. Rats infected with T. solium AO and PO forms developed neurocysticercosis (NCC), while those infected with the T. saginata did not. Human PMBCs were stimulated with antigens of AO and PO forms of both species, and the production of cytokines and metalloproteinases (MMPs) was measured. The T. solium AO antigen stimulated a higher production of IL-4, IL-5, IL-13, IFN-γ, and IL-2 cytokines compared to T. saginata AO. In the PO form, the T. saginata PO antigen increased the production of IL-4, IL-5, IL-13, IFN-γ, IL-1β, IL-6, IL-10, TNF-α and IL-12 cytokines compared to T. solium, suggesting that this global immune response stimulated by different forms could permit survival or destruction of the parasite depending of their life-cycle stage. Regarding MMPs, T. solium AO antigen stimulated a higher production of MMP-9 compared to T. saginata AO antigen, which may be responsible for altering the permeability of intestinal cells and facilitating breakdown of the blood-brain barrier during the process of invasion of host tissue.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



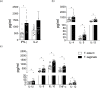

References
-
- Global Health—Division of Parasitic Diseases. CDC—Taeniasis—Enfermedad [Internet]. Centers Dis. Control Prev. 2013. [cited 2018 Sep 14];Available from: https://www.cdc.gov/parasites/taeniasis/es/enfermedad.html
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

