Peripheral blood-derived mesenchymal stem cells demonstrate immunomodulatory potential for therapeutic use in horses
- PMID: 30870461
- PMCID: PMC6417789
- DOI: 10.1371/journal.pone.0212642
Peripheral blood-derived mesenchymal stem cells demonstrate immunomodulatory potential for therapeutic use in horses
Abstract
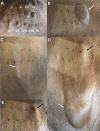
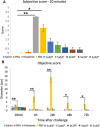
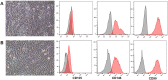
Previously, we showed that mesenchymal stem cells (MSC) can be mobilized into peripheral blood using electroacupuncture (EA) at acupoints, LI-4, LI-11, GV-14, and GV-20. The purpose of this study was to determine whether EA-mobilized MSC could be harvested and expanded in vitro to be used as an autologous cell therapy in horses. Peripheral blood mononuclear cells (PBMC) isolated from young and aged lame horses (n = 29) showed a marked enrichment for MSCs. MSC were expanded in vitro (n = 25) and administered intravenously at a dose of 50 x 106 (n = 24). Treatment resulted in significant improvement in lameness as assessed by the American Association of Equine Practitioners (AAEP) lameness scale (n = 23). MSCs exhibited immunomodulatory function by inhibition of lymphocyte proliferation and induction of IL-10. Intradermal testing showed no immediate or delayed immune reactions to MSC (1 x 106 to 1 x 104). In this study, we demonstrated an efficient, safe and reproducible method to mobilize and expand, in vitro, MSCs in sufficiently high concentrations for therapeutic administration. We confirm the immunomodulatory function of these cells in vitro. This non-pharmacological and non-surgical strategy for stem cell harvest has a broad range of biomedical applications and represents an improved clinically translatable and economical cell source for humans.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Crovace A, Lacitignola L, Rossi G, Francioso E. Histological and immunohistochemical evaluation of autologous cultured bone marrow mesenchymal stem cells and bone marrow mononucleated cells in collagenase-induced tendinitis of equine superficial digital flexor tendon. Vet Med Int. 2010;2010:250978 10.4061/2010/250978 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

