Ultra-performance hydrophilic interaction liquid chromatography coupled with tandem mass spectrometry for simultaneous determination of allopurinol, oxypurinol and lesinurad in rat plasma: Application to pharmacokinetic study in rats
- PMID: 30870504
- PMCID: PMC6417734
- DOI: 10.1371/journal.pone.0213786
Ultra-performance hydrophilic interaction liquid chromatography coupled with tandem mass spectrometry for simultaneous determination of allopurinol, oxypurinol and lesinurad in rat plasma: Application to pharmacokinetic study in rats
Abstract
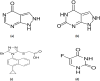
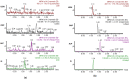
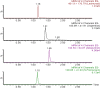
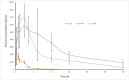
A fixed dose combination of lesinurad and allopurinol has been recently approved by USFDA and EMA for treatment of gout-associated hyperuricemia in patients who have not achieved target serum uric acid levels with allopurinol alone. In this study, an ultra-performance hydrophilic interaction liquid chromatography (UPHILIC) coupled with tandem mass spectrometry method was developed and validated for simultaneous determination of allopurinol, oxypurinol and lesinurad in rat plasma. Liquid liquid extraction using ethyl acetate as extracting agent was used for samples extraction procedure. Acquity UPLC HILIC column (100 mm x 2.1, 1.7μm) was used for separation of allopurinol, oxypurinol, lesinurad and internal standard (5-Florouracil). The mobile phase consisting of acetonitrile, water and formic acid (95:5:0.1, v/v/v), were eluted at 0.3 mL/min flow rate having total chromatographic run time of 3 min per sample. The analytes were detected on Acquity triple quadrupole mass spectrometer equipped with a Z-Spray electrospray ionization (ESI). The ESI source was operated in negative mode and multiple reaction monitoring was used for ion transition for all compounds. The precursor to product ion transition of m/z 134.94 > 64.07 for allopurinol, 150.89 > 41.91 for oxypurinol, 401.90 > 176.79 for lesinurad and 128.85 >41.92 for internal standard were used for identification and quantification. The calibration curves for all analytes were found to be linear with weighing factor of 1/x2 using regression analysis. The developed assay was successfully applied in an oral pharmacokinetic study of allopurinol, oxypurinol and lesinurad in rats.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Determination of allopurinol and oxypurinol in human plasma and urine by liquid chromatography-tandem mass spectrometry.J Chromatogr B Analyt Technol Biomed Life Sci. 2013 Dec 15;941:10-6. doi: 10.1016/j.jchromb.2013.09.028. Epub 2013 Oct 1. J Chromatogr B Analyt Technol Biomed Life Sci. 2013. PMID: 24184830
-
The Effects of Special Patient Population Plasma on Pharmacokinetic Quantifications Using LC-MS/MS.Drug Metab Lett. 2019;13(2):111-122. doi: 10.2174/1872312813666191015162634. Drug Metab Lett. 2019. PMID: 31613735
-
Measurement of urinary oxypurinol by high performance liquid chromatography-tandem mass spectrometry.J Chromatogr B Analyt Technol Biomed Life Sci. 2010 Sep 1;878(25):2363-8. doi: 10.1016/j.jchromb.2010.07.017. Epub 2010 Jul 29. J Chromatogr B Analyt Technol Biomed Life Sci. 2010. PMID: 20702150
-
UPLC-MS/MS determination and gender-related pharmacokinetic study of five active ingredients in rat plasma after oral administration of Eucommia cortex extract.J Ethnopharmacol. 2015 Jul 1;169:145-55. doi: 10.1016/j.jep.2015.04.007. Epub 2015 Apr 22. J Ethnopharmacol. 2015. PMID: 25910535
-
Lesinurad.2019 May 1. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. 2019 May 1. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. PMID: 31643315 Free Books & Documents. Review.
Cited by
-
Mesoporous Silica Nanoparticles Coated with Carboxymethyl Chitosan for 5-Fluorouracil Ocular Delivery: Characterization, In Vitro and In Vivo Studies.Molecules. 2023 Jan 27;28(3):1260. doi: 10.3390/molecules28031260. Molecules. 2023. PMID: 36770926 Free PMC article.
-
Mechanosynthesis of Stable Salt Hydrates of Allopurinol with Enhanced Dissolution, Diffusion, and Pharmacokinetics.ACS Omega. 2023 Sep 7;8(37):34120-34133. doi: 10.1021/acsomega.3c05263. eCollection 2023 Sep 19. ACS Omega. 2023. PMID: 37744830 Free PMC article.
-
Simultaneous determination of lesinurad and co-administered drugs used in management of gout comorbidities to uncover potential pharmacokinetic interaction in rat plasma.Sci Rep. 2025 Apr 2;15(1):11238. doi: 10.1038/s41598-025-93680-4. Sci Rep. 2025. PMID: 40175395 Free PMC article.
-
A Brief Review of Analytical Methods for the Estimation of Allopurinol in Pharmaceutical Formulation and Biological Matrices.Int J Anal Chem. 2021 Jun 5;2021:5558651. doi: 10.1155/2021/5558651. eCollection 2021. Int J Anal Chem. 2021. PMID: 34194505 Free PMC article. Review.
References
-
- Yue TF and Gutman AB. Effect of allopurinol (4-hydroxypyrazolo-(3,4-d)pyrimidine) on serum and urinary uric acid in primary and secondary gout. Am J Med. 1964; 37:885–898. - PubMed
-
- Turnheim K, Krivanek P, Oberbauer R. Pharmacokinetics and pharmacodynamics of allopurinol in elderly and young subjects.Br J Clin Pharmacol. 1999; 48(4):501–509. 10.1046/j.1365-2125.1999.00041.x - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

