ERBB2-modulated ATG4B and autophagic cell death in human ARPE19 during oxidative stress
- PMID: 30870514
- PMCID: PMC6417729
- DOI: 10.1371/journal.pone.0213932
ERBB2-modulated ATG4B and autophagic cell death in human ARPE19 during oxidative stress
Abstract
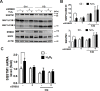
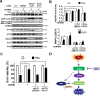
Age-related macular degeneration (AMD) is an ocular disease with retinal degeneration. Retinal pigment epithelium (RPE) degeneration is mainly caused by long-term oxidative stress. Kinase activity could be either protective or detrimental to cells during oxidative stress; however, few reports have described the role of kinases in oxidative stress. In this study, high-throughput screening of kinome siRNA library revealed that erb-b2 receptor tyrosine-protein kinase 2 (ERBB2) knockdown reduced reactive oxygen species (ROS) production in ARPE-19 cells during oxidative stress. Silencing ERBB2 caused an elevation in microtubule associated protein light chain C3-II (MAP1LC3B-II/I) conversion and sequesterone (SQSTM)1 protein level. ERBB2 deprivation largely caused an increase in autophagy-regulating protease (ATG4B) expression, a protease that negatively recycles MAP1LC3-II at the fusion step between the autophagosome and lysosome, suggesting ERBB2 might modulate ATG4B for autophagy induction in oxidative stress-stimulated ARPE-19 cells. ERBB2 knockdown also caused an accumulation of nuclear factor erythroid 2-related factor 2 (NRF2) and enhanced its transcriptional activity. In addition, ERBB2 ablation or treatment with autophagy inhibitors reduced oxidative-induced cytotoxic effects in ARPE-19 cells. Furthermore, ERBB2 silencing had little or no additive effects in ATG5/7-deficient cells. Taken together, our results suggest that ERBB2 may play an important role in modulating autophagic RPE cell death during oxidative stress, and ERBB2 may be a potential target in AMD prevention.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


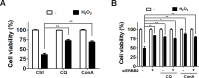
Similar articles
-
Protective effect of canolol from oxidative stress-induced cell damage in ARPE-19 cells via an ERK mediated antioxidative pathway.Mol Vis. 2011;17:2040-8. Epub 2011 Jul 27. Mol Vis. 2011. PMID: 21850179 Free PMC article.
-
Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD.Autophagy. 2014;10(11):1989-2005. doi: 10.4161/auto.36184. Autophagy. 2014. PMID: 25484094 Free PMC article.
-
All-trans-retinal induces autophagic cell death via oxidative stress and the endoplasmic reticulum stress pathway in human retinal pigment epithelial cells.Toxicol Lett. 2020 Apr 1;322:77-86. doi: 10.1016/j.toxlet.2020.01.005. Epub 2020 Jan 10. Toxicol Lett. 2020. PMID: 31931077
-
Nrf2 signaling is impaired in the aging RPE given an oxidative insult.Exp Eye Res. 2014 Feb;119:111-4. doi: 10.1016/j.exer.2013.10.024. Epub 2013 Nov 8. Exp Eye Res. 2014. PMID: 24216314 Free PMC article. Review.
-
The Regulation of NFE2L2 (NRF2) Signalling and Epithelial-to-Mesenchymal Transition in Age-Related Macular Degeneration Pathology.Int J Mol Sci. 2019 Nov 18;20(22):5800. doi: 10.3390/ijms20225800. Int J Mol Sci. 2019. PMID: 31752195 Free PMC article. Review.
Cited by
-
The interplay of autophagy and oxidative stress in the pathogenesis and therapy of retinal degenerative diseases.Cell Biosci. 2022 Jan 3;12(1):1. doi: 10.1186/s13578-021-00736-9. Cell Biosci. 2022. PMID: 34980273 Free PMC article. Review.
-
The Pathomechanism, Antioxidant Biomarkers, and Treatment of Oxidative Stress-Related Eye Diseases.Int J Mol Sci. 2022 Jan 23;23(3):1255. doi: 10.3390/ijms23031255. Int J Mol Sci. 2022. PMID: 35163178 Free PMC article. Review.
-
Association of ATG4B and Phosphorylated ATG4B Proteins with Tumorigenesis and Prognosis in Oral Squamous Cell Carcinoma.Cancers (Basel). 2019 Nov 23;11(12):1854. doi: 10.3390/cancers11121854. Cancers (Basel). 2019. PMID: 31771238 Free PMC article.
-
Topical Ascorbic Acid Ameliorates Oxidative Stress-Induced Corneal Endothelial Damage via Suppression of Apoptosis and Autophagic Flux Blockage.Cells. 2020 Apr 11;9(4):943. doi: 10.3390/cells9040943. Cells. 2020. PMID: 32290365 Free PMC article.
-
Detection of Autophagy-Related Gene Expression by Conjunctival Impression Cytology in Age-Related Macular Degeneration.Diagnostics (Basel). 2021 Feb 12;11(2):296. doi: 10.3390/diagnostics11020296. Diagnostics (Basel). 2021. PMID: 33673354 Free PMC article.
References
-
- Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. The Lancet Global health. 2014;2(2):e106–16. Epub 2014/08/12. 10.1016/S2214-109X(13)70145-1 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

