Varicella zoster virus productively infects human peripheral blood mononuclear cells to modulate expression of immunoinhibitory proteins and blocking PD-L1 enhances virus-specific CD8+ T cell effector function
- PMID: 30870532
- PMCID: PMC6435197
- DOI: 10.1371/journal.ppat.1007650
Varicella zoster virus productively infects human peripheral blood mononuclear cells to modulate expression of immunoinhibitory proteins and blocking PD-L1 enhances virus-specific CD8+ T cell effector function
Abstract
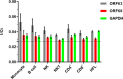
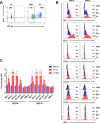
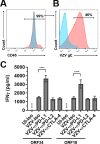
Varicella zoster virus (VZV) is a lymphotropic alpha-herpesvirinae subfamily member that produces varicella on primary infection and causes zoster, vascular disease and vision loss upon reactivation from latency. VZV-infected peripheral blood mononuclear cells (PBMCs) disseminate virus to distal organs to produce clinical disease. To assess immune evasion strategies elicited by VZV that may contribute to dissemination of infection, human PBMCs and VZV-specific CD8+ T cells (V-CD8+) were mock- or VZV-infected and analyzed for immunoinhibitory protein PD-1, PD-L1, PD-L2, CTLA-4, LAG-3 and TIM-3 expression using flow cytometry. All VZV-infected PBMCs (monocytes, NK, NKT, B cells, CD4+ and CD8+ T cells) and V-CD8+ showed significant elevations in PD-L1 expression compared to uninfected cells. VZV induced PD-L2 expression in B cells and V-CD8+. Only VZV-infected CD8+ T cells, NKT cells and V-CD8+ upregulated PD-1 expression, the immunoinhibitory receptor for PD-L1/PD-L2. VZV induced CTLA-4 expression only in V-CD8+ and no significant changes in LAG-3 or TIM-3 expression were observed in V-CD8+ or PBMC T cells. To test whether PD-L1, PD-L2 or CTLA-4 regulates V-CD8+ effector function, autologous PBMCs were VZV-infected and co-cultured with V-CD8+ cells in the presence of blocking antibodies against PD-L1, PD-L2 or CTLA-4; ELISAs revealed significant elevations in IFNγ only upon blocking of PD-L1. Together, these results identified additional immune cells that are permissive to VZV infection (monocytes, B cells and NKT cells); along with a novel mechanism for inhibiting CD8+ T cell effector function through induction of PD-L1 expression.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Schmader K, George LK, Burchett BM, Pieper CF, Hamilton JD. Racial differences in the occurrence of herpes zoster. J Infect Dis. 1995;171(3):701–4. Epub 1995/03/01. . - PubMed
-
- Matsukura H, Murakami M, Sakaki H, Mitani T, Shimura S. Varicella glomerulonephritis preceding the cutaneous lesions. Clin Nephrol. 2009;72(2):161–2. Epub 2009/07/31. . - PubMed
-
- Mantadakis E, Anagnostatou N, Danilatou V, Markaki EA, Spanaki AM, Briassoulis G, et al. Fulminant hepatitis due to varicella zoster virus in a girl with acute lymphoblastic leukemia in remission: report of a case and review. J Pediatr Hematol Oncol. 2005;27(10):551–3. Epub 2005/10/12. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

