Detection of Inflammation-Related Melanoma Small Extracellular Vesicle (sEV) mRNA Content Using Primary Melanocyte sEVs as a Reference
- PMID: 30870978
- PMCID: PMC6429302
- DOI: 10.3390/ijms20051235
Detection of Inflammation-Related Melanoma Small Extracellular Vesicle (sEV) mRNA Content Using Primary Melanocyte sEVs as a Reference
Abstract
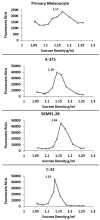
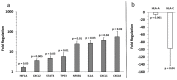
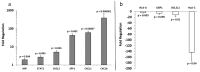
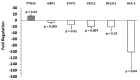
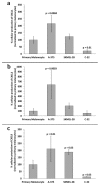
Melanoma-derived small extracellular vesicles (sEVs) participate in tumor pathogenesis. Tumor pathogenesis is highly dependent on inflammatory processes. Given the potential for melanoma sEVs to carry tumor biomarkers, we explored the hypothesis that they may contain inflammation-related mRNA content. Biophysical characterization showed that human primary melanocyte-derived sEVs trended toward being smaller and having less negative (more neutral) zeta potential than human melanoma sEVs (A-375, SKMEL-28, and C-32). Using primary melanocyte sEVs as the control population, RT-qPCR array results demonstrated similarities and differences in gene expression between melanoma sEV types. Upregulation of pro-angiogenic chemokine ligand CXCL1, CXCL2, and CXCL8 mRNAs in A-375 and SKMEL-28 melanoma sEVs was the most consistent finding. This paralleled increased production of CXCL1, CXCL2, and CXCL8 proteins by A-375 and SKMEL-28 sEV source cells. Overall, the use of primary melanocyte sEVs as a control sEV reference population facilitated the detection of inflammation-related melanoma sEV mRNA content.
Keywords: biomarker; exosome; extracellular vesicle; inflammation; mRNA; melanoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Thery C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., et al. Minimal information for studies of extracellular vesicles 2018 (misev2018): A position statement of the international society for extracellular vesicles and update of the misev2014 guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. - DOI - PMC - PubMed
-
- Kowal J., Arras G., Colombo M., Jouve M., Morath J.P., Primdal-Bengtson B., Dingli F., Loew D., Tkach M., Thery C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA. 2016;113:E968–E977. doi: 10.1073/pnas.1521230113. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

