CXCR2: A Novel Mediator of Mammary Tumor Bone Metastasis
- PMID: 30871004
- PMCID: PMC6429058
- DOI: 10.3390/ijms20051237
CXCR2: A Novel Mediator of Mammary Tumor Bone Metastasis
Abstract
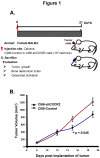
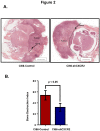
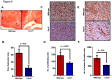
Most breast cancer patients die due to bone metastasis. Although metastasis accounts for 5% of the breast cancer cases, it is responsible for most of the deaths. Sometimes even before the detection of a primary tumor, most of the patients have bone and lymph node metastasis. Moreover, at the time of death, breast cancer patients have the bulk of the tumor burden in their bones. Therapy options are available for the treatment of primary tumors, but there are minimal options for treating breast cancer patients who have bone metastasis. C-X-C motif chemokine receptor type 2 (CXCR2) receptor-mediated signaling has been shown to play a critical role during bone-related inflammations and its ligands C-X-C motif chemokine ligand 6 (CXCL6) and 8 (CXCL8) aid in the resorption of bone during bone metastasis. In this study, we tested the hypothesis that CXCR2 contributes to mammary tumor-induced osteolysis and bone metastasis. In the present study, we examined the role of both tumor cell-derived and host-derived CXCR2 in influencing mammary tumor cell bone metastasis. For understanding the role of tumor cell-derived CXCR2, we utilized Cl66 CXCR2 knockdown (Cl66-shCXCR2) and Cl66-Control cells (Cl66-Control) and observed a significant decrease in tumor growth and tumor-induced osteolysis in Cl66-shCXCR2 cells in comparison with the Cl66-Control cells. Next, for understanding the role of host-derived CXCR2, we utilized mice with genomic knockdown of CXCR2 (Cxcr2-/-) and injected Cl66-Luciferase (Cl66-Luc) or 4T1-Luciferase (4T1-Luc) cells. We observed decreased bone destruction and metastasis in the bone of Cxcr2-/- mice. Our data suggest the importance of both tumor cell- and host-derived CXCR2 signaling in the bone metastasis of breast cancer cells.
Keywords: CXCR2; bone microenvironment; metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Benoy I.H., Salgado R., Van Dam P., Geboers K., Van Marck E., Scharpe S., Vermeulen P.B., Dirix L.Y. Increased Serum Interleukin-8 in Patients with Early and Metastatic Breast Cancer Correlates with Early Dissemination and Survival. Clin. Cancer Res. 2004;10:7157–7162. doi: 10.1158/1078-0432.CCR-04-0812. - DOI - PubMed
-
- Kozlowski L., Zakrzewska I., Tokajuk P., Wojtukiewicz M.Z. Concentration of interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of breast cancer patients. Rocz. Akad. Med. Bialymst. 2003;48:82–84. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

