Increased iNOS and Nitrosative Stress in Dopaminergic Neurons of MDMA-Exposed Rats
- PMID: 30871034
- PMCID: PMC6429174
- DOI: 10.3390/ijms20051242
Increased iNOS and Nitrosative Stress in Dopaminergic Neurons of MDMA-Exposed Rats
Abstract
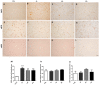
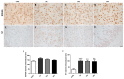
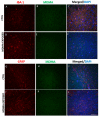
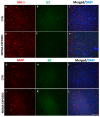
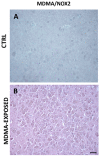
Several mechanisms underlying 3,4-Methylenedioxy-N-methylamphetamine (MDMA) neurotoxicity have been proposed, including neurochemical alterations and excitotoxicity mediated by reactive oxygen species (ROS), nitric oxide (NO), and reactive nitrogen species (RNS). However, ROS, NO, and RNS sources in the brain are not fully known. We aimed to investigate possible alterations in the expression of the ROS producer NOX enzymes (NOX2, NOX1, and NOX4), NO generators (iNOS, eNOS, and nNOS), markers of oxidative (8-hydroxy-2'-deoxyguanosine, 8OHdG), and nitrosative (3-nitrotyrosine, NT) stress, as well as the colocalization between cells positive for the dopamine transporter (DT1) and cells expressing the neuronal nuclei (NeuN) marker, in the frontal cortex of rats receiving saline or MDMA, sacrificed 6 h, 16 h, or 24 h after its administration. MDMA did not affect NOX2, NOX1, and NOX4 immunoreactivity, whereas iNOS expression was enhanced. The number of NT-positive cells was increased in MDMA-exposed animals, whereas no differences were detected in 8OHdG expression among experimental groups. MDMA and NT markers colocalized with DT1 positive cells. DT1 immunostaining was found in NeuN-positive stained cells. Virtually no colocalization was observed with microglia and astrocytes. Moreover, MDMA immunostaining was not found in NOX2-positive cells. Our results suggest that iNOS-derived nitrosative stress, but not NOX enzymes, may have a crucial role in the pathogenesis of MDMA-induced neurotoxicity, highlighting the specificity of different enzymatic systems in the development of neuropathological alterations induced by the abuse of this psychoactive compound.
Keywords: MDMA; NADPH oxidases; iNOS; nitrosative stress; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

