Sofosbuvir-based regimen is safe and effective for hepatitis C infected patients with stage 4-5 chronic kidney disease: a systematic review and meta-analysis
- PMID: 30871566
- PMCID: PMC6419462
- DOI: 10.1186/s12985-019-1140-x
Sofosbuvir-based regimen is safe and effective for hepatitis C infected patients with stage 4-5 chronic kidney disease: a systematic review and meta-analysis
Abstract
Background: Whether sofosbuvir is suitable for hepatitis C virus (HCV) infected patients with severe renal impairment is inconclusive. This systematic review aims to evaluate the safety and effectiveness of SOF-based regimen in the setting of stage 4 and 5 chronic kidney disease (CKD).
Methods: We conducted a systematic literature search in PubMed, Web of Science, EMBASE and Google Scholar with searching strategy: (sofosbuvir OR Sovaldi OR Harvoni OR Epclusa OR Vosevi) AND (severe kidney impairment OR severe renal impairment OR end-stage renal disease OR dialysis OR renal failure OR ESRD OR renal insufficiency OR hepatorenal syndrome OR HRS). Sustained virological response (SVR12/24) rate and serious adverse event (SAE) rate with 95% confidence intervals were aggregated. Subgroup analysis was implemented to evaluate the impact of treatment strategy and patient characteristics.
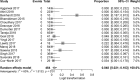
Results: Twenty-one studies met inclusion criteria, totaling 717 HCV infected patients with CKD stage 4 or 5 (58.4% on dialysis). Pooled SVR12/24 was 97.1% (95% CI 93.9-99.3%), and SAE rate was 4.8% (95% CI 2.1-10.3%). There was no significant difference at SVR12/24 (97.1% vs 96.2%, p = 0.72) or SAE rate (8.8% vs 2.9%, p = 0.13) between subgroups applying full or decreased dose of sofosbuvir. Cirrhotic and non-cirrhotic patients achieved comparable sustained virological response (RR 0.93, 95% CI 0.85-1.02). Four studies reported eGFR/serum creatinine pre- and post- treatment, with no significant modification.
Conclusions: Our study suggests SOF-based regimen might be used safely and effectively in patients living with HCV infection/stage 4-5 CKD, with normal and reduced dose of sofosbuvir. Prospective and well-controlled trials are needed to confirm these findings.
Trial registration: PROSPERO CRD42018107440 .
Keywords: Dose; HCV infection; Meta-analysis; Sofosbuvir; Stage 4–5 chronic kidney disease.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
MSL works for Gilead while concurrently enrolled in Ph.D.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
Sofosbuvir-based regimens for HCV in stage 4-stage 5 chronic kidney disease. A systematic review with meta-analysis.Nefrologia (Engl Ed). 2021 Sep-Oct;41(5):578-589. doi: 10.1016/j.nefroe.2021.11.011. Nefrologia (Engl Ed). 2021. PMID: 36165141
-
Efficacy and safety of sofosbuvir-based regimens for treatment in chronic hepatitis C genotype 1 patients with moderately impaired renal function.Clin Mol Hepatol. 2017 Dec;23(4):316-322. doi: 10.3350/cmh.2016.0087. Epub 2017 Aug 22. Clin Mol Hepatol. 2017. PMID: 28827512 Free PMC article.
-
Sofosbuvir plus ribavirin and sofosbuvir plus ledipasvir in patients with genotype 1 or 3 hepatitis C virus and severe renal impairment: a multicentre, phase 2b, non-randomised, open-label study.Lancet Gastroenterol Hepatol. 2020 Oct;5(10):918-926. doi: 10.1016/S2468-1253(19)30417-0. Epub 2020 Jun 10. Lancet Gastroenterol Hepatol. 2020. PMID: 32531259 Clinical Trial.
-
An updated systematic review and meta-analysis on efficacy of Sofosbuvir in treating hepatitis C-infected patients with advanced chronic kidney disease.PLoS One. 2021 Feb 10;16(2):e0246594. doi: 10.1371/journal.pone.0246594. eCollection 2021. PLoS One. 2021. PMID: 33566846 Free PMC article.
-
Sofosbuvir plus velpatasvir combination for the treatment of chronic hepatitis C in patients with end stage renal disease on renal replacement therapy: A systematic review and meta-analysis.Nephrology (Carlton). 2022 Jan;27(1):82-89. doi: 10.1111/nep.13968. Epub 2021 Sep 14. Nephrology (Carlton). 2022. PMID: 34453374
Cited by
-
Decade of optimizing therapy with direct-acting antiviral drugs and the changing profile of patients with chronic hepatitis C.World J Gastroenterol. 2023 Feb 14;29(6):949-966. doi: 10.3748/wjg.v29.i6.949. World J Gastroenterol. 2023. PMID: 36844142 Free PMC article. Review.
-
Management of Hepatitis C Virus and Hepatitis B Virus Infection in the Setting of Kidney Disease.Adv Kidney Dis Health. 2023 Jul;30(4):343-355. doi: 10.1053/j.akdh.2023.04.003. Adv Kidney Dis Health. 2023. PMID: 37657881 Free PMC article. Review.
-
Sofosbuvir and risk of estimated glomerular filtration rate decline or end-stage renal disease in patients with renal impairment.Aliment Pharmacol Ther. 2022 May;55(9):1169-1178. doi: 10.1111/apt.16830. Epub 2022 Mar 2. Aliment Pharmacol Ther. 2022. PMID: 35235245 Free PMC article. Clinical Trial.
-
Immediate administration of antiviral therapy after transplantation of hepatitis C-infected livers into uninfected recipients: Implications for therapeutic planning.Am J Transplant. 2020 Jun;20(6):1619-1628. doi: 10.1111/ajt.15768. Epub 2020 Feb 3. Am J Transplant. 2020. PMID: 31887236 Free PMC article.
-
Serum Neutrophil Gelatinase-Associated Lipocalin (NGAL) in HCV-Positive Egyptian Patients Treated with Sofosbuvir.Can J Gastroenterol Hepatol. 2020 Jan 27;2020:1632959. doi: 10.1155/2020/1632959. eCollection 2020. Can J Gastroenterol Hepatol. 2020. PMID: 32083035 Free PMC article.
References
-
- WHO . GUIDELINES FOR THE CARE AND TREATMENT OF PERSONS DIAGNOSED WITH CHRONIC HEPATITIS C VIRUS INFECTION. 2018. - PubMed
-
- Fissell RB, Bragg-Gresham JL, Woods JD, Jadoul M, Gillespie B, Hedderwick SA, Rayner HC, Greenwood RN, Akiba T, Young EW. Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: the DOPPS. Kidney Int. 2004;65(6):2335–2342. doi: 10.1111/j.1523-1755.2004.00649.x. - DOI - PubMed
-
- Fabrizi F, Takkouche B, Lunghi G, Dixit V, Messa P, Martin P. The impact of hepatitis C virus infection on survival in dialysis patients: meta-analysis of observational studies. J Viral Hepat. 2007;14(10):697–703. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

