Loss of NF-κB p50 function synergistically augments microglial priming in the middle-aged brain
- PMID: 30871598
- PMCID: PMC6419422
- DOI: 10.1186/s12974-019-1446-z
Loss of NF-κB p50 function synergistically augments microglial priming in the middle-aged brain
Abstract
Background: While NF-κB p50 function is impaired in central nervous system disease, aging in non-CNS tissues, and response to reactive oxygen species, the role of NF-κB p50 in aging-associated microglial pro-inflammatory priming is poorly understood.
Methods: Male NF-κB p50+/+ and NF-κB p50-/- mice at three different ages (1.5-3.0 month old, 8.0-11.0 month old, and 16.0-18.0 month old) were treated with LPS (5 mg/kg, IP) to trigger peripheral inflammation, where circulating cytokines, neuroinflammation, microglia morphology, and NF-κB p50/p65 function in brain tissue were determined 3 h later.
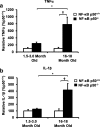
Results: Peripheral LPS injection in 9-month-old C57BL/6 mice resulted in lower NF-κB p50 DNA binding of nuclear extracts from the whole brain, when compared to 3-week-old C57BL/6 mice, revealing differences in LPS-induced NF-κB p50 activity in the brain across the mouse lifespan. To examine the consequences of loss NF-κB p50 function with aging, NF-κB p50+/+ and NF-κB p50-/- mice of three different age groups (1.5-3.0 month old, 8.0-11.0 month old, and 16.0-18.0 month old) were injected with LPS (5 mg/kg, IP). NF-κB p50-/- mice showed markedly elevated circulating, midbrain, and microglial TNFα when compared to NF-κB p50+/+ mice at all ages. Notably, the 16.0-18.0-month-old (middle aged) NF-κB p50-/- mice exhibited synergistically augmented LPS-induced serum and midbrain TNFα when compared to the younger (1.5-3.0 month old, young adult) NF-κB p50-/- mice. The 16.0-18.0-month-old LPS-treated NF-κB p50-/- mice also had the highest midbrain IL-1β expression, largest number of microglia with changes in morphology, and greatest elevation of pro-inflammatory factors in isolated adult microglia. Interestingly, aging NF-κB p50-/- mice exhibited decreased brain NF-κB p65 expression and activity.
Conclusions: These findings support that loss of NF-κB p50 function and aging in middle-aged mice may interact to excessively augment peripheral/microglial pro-inflammatory responses and point to a novel neuroinflammation signaling mechanism independent the NF-κB p50/p65 transcription factor in this process.
Keywords: Aging; Microglia; NF-κB; Priming.
Conflict of interest statement
Ethics approval and consent to participate
This research was performed within the strict NIH ethics and guidelines with the approval of the IUSM IACUC committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

