The effects and safety of vasopressin receptor agonists in patients with septic shock: a meta-analysis and trial sequential analysis
- PMID: 30871607
- PMCID: PMC6419432
- DOI: 10.1186/s13054-019-2362-4
The effects and safety of vasopressin receptor agonists in patients with septic shock: a meta-analysis and trial sequential analysis
Abstract
Background: The aim of this study was to evaluate the effects and safety of vasopressin receptor agonists in patients with septic shock.
Methods: PubMed, EMBASE, and Cochrane library were searched for randomized controlled trials evaluating the effects of vasopressin receptor agonists in septic shock patients. Two reviewers performed literature selection, data extraction, and quality evaluation independently. The primary outcome was mortality. And secondary outcomes included intensive care unit (ICU) length of stay, duration of mechanical ventilation, and incidence of adverse events. In addition, a trial sequential analysis (TSA) was performed.
Results: Twenty studies were eligible for meta-analysis. The results showed vasopressin receptor agonists use was associated with reduced mortality (relative risk (RR) 0.92; 95% confidence interval (CI) 0.84 to 0.99; I2 = 0%). Nevertheless, they had no significant effects on ICU length of stay (mean deviation (MD) - 0.08, 95% CI, - 0.68 to 0.52, I2 = 0%) and duration of mechanical ventilation (MD - 0.58, 95% CI - 1.47 to 0.31, I2 = 57%). Additionally, there was no significant difference in total adverse events between two groups (RR 1.28, 95% CI 0.87 to 1.90, I2 = 57%), but vasopressin receptor agonists administration could significantly increase the risk of digital ischemia (RR 4.85, 95% CI 2.81 to 8.39, I2 = 26%). Finally, there was no statistical difference of cardiovascular events (RR 0.91, 95% CI 0.53 to 1.57, I2 = 1%), arrhythmia (0.77, 95% CI 0.48 to 1.23, I2 = 23%), mesenteric ischemia (0.83, 95% CI 0.44 to 1.55, I2 = 0%), diarrhea (2.47, 95% CI 0.77 to 7.96, I2 = 49%), cerebrovascular events (1.36, 95% CI 0.18 to 10.54, I2 = 0%), and hyponatremia (1.47, 95% CI 0.84 to 2.55, I2 = 0%) between two groups. Egger's test showed there was no significant publication bias among studies (P = 0.36).
Conclusions: The use of vasopressin might result in reduced mortality in patients with septic shock. An increased risk of digital ischemia must be taken into account.
Keywords: Catecholamine; Meta-analysis; Septic shock; Vasopressin.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
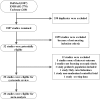



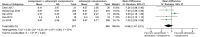


References
-
- Machado FR, Cavalcanti AB, Bozza FA, Ferreira EM, Angotti Carrara FS, Sousa JL, Caixeta N, Salomao R, Angus DC, Pontes Azevedo LC. The epidemiology of sepsis in Brazilian intensive care units (the Sepsis PREvalence Assessment Database, SPREAD): an observational study. Lancet Infect Dis. 2017;17(11):1180–1189. - PubMed
-
- SepNet Critical Care Trials Group. Incidence of severe sepsis and septic shock in German intensive care units: the prospective, multicentre INSEP study. Intensive Care Med. 2016;42(12):1980-9. - PubMed
-
- Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, Weiss YG, Benbenishty J, Kalenka A, Forst H, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358(2):111–124. - PubMed
-
- Maas JJ, Pinsky MR, de Wilde RB, de Jonge E, Jansen JR. Cardiac output response to norepinephrine in postoperative cardiac surgery patients: interpretation with venous return and cardiac function curves. Crit Care Med. 2013;41(1):143–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

