Cocultures of human colorectal tumor spheroids with immune cells reveal the therapeutic potential of MICA/B and NKG2A targeting for cancer treatment
- PMID: 30871626
- PMCID: PMC6417026
- DOI: 10.1186/s40425-019-0553-9
Cocultures of human colorectal tumor spheroids with immune cells reveal the therapeutic potential of MICA/B and NKG2A targeting for cancer treatment
Abstract
Background: Immunotherapies still fail to benefit colorectal cancer (CRC) patients. Relevant functional assays aimed at studying these failures and the efficacy of cancer immunotherapy in human are scarce. 3D tumor cultures, called tumor organoids or spheroids, represent interesting models to study cancer treatments and could help to challenge these issues.
Methods: We analyzed heterotypic cocultures of human colon tumor-derived spheroids with immune cells to assess the infiltration, activation and function of T and NK cells toward human colorectal tumors in vitro.
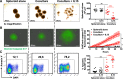
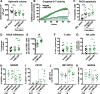
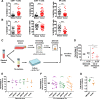
Results: We showed that allogeneic T and NK cells rapidly infiltrated cell line-derived spheroids, inducing immune-mediated tumor cell apoptosis and spheroid destruction. NKG2D, a key activator of cytotoxic responses, was engaged on infiltrating cells. We thus assessed the therapeutic potential of an antibody targeting the specific ligands of NKG2D, MICA and MICB, in this system. Anti-MICA/B enhanced immune-dependent destruction of tumor spheroid by driving an increased NK cells infiltration and activation. Interestingly, tumor cells reacted to immune infiltration by upregulating HLA-E, ligand of the inhibitory receptor NKG2A expressed by CD8 and NK cells. NKG2A was increased after anti-MICA/B treatment and, accordingly, combination of anti-MICA/B and anti-NKG2A was synergistic. These observations were ultimately confirmed in a clinical relevant model of coculture between CRC patients-derived spheroids and autologous tumor-infiltrating lymphocytes.
Conclusions: Altogether, we show that tumor spheroids represent a relevant tool to study tumor-lymphocyte interactions on human tissues and revealed the antitumor potential of immunomodulatory antibodies targeting MICA/B and NKG2A.
Keywords: Colorectal cancer; Immunotherapy; MICA/B; NKG2A; Spheroids.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the French ethical committee (approval n°2016/45), and all subjects gave written informed consent.
Consent for publication
Not applicable
Competing interests
RR, LAM and MB are employees of Innate Pharma. This work was partly supported by a collaborative grant obtained from Innate Pharma by TA, MA and LLB.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Golstein P, Griffiths GM. An early history of T cell-mediated cytotoxicity. Nat Rev Immunol. 2018;18(8):527–35. - PubMed
-
- Melero I, Rouzaut A, Motz GT, Coukos G. T-cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious Cancer immunotherapy. Cancer Discov. 2014;4:522–526. doi: 10.1158/2159-8290.CD-13-0985. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
