Detection and Prediction of Bioprosthetic Aortic Valve Degeneration
- PMID: 30871693
- PMCID: PMC6424589
- DOI: 10.1016/j.jacc.2018.12.056
Detection and Prediction of Bioprosthetic Aortic Valve Degeneration
Abstract
Background: Bioprosthetic aortic valve degeneration is increasingly common, often unheralded, and can have catastrophic consequences.
Objectives: The authors sought to assess whether 18F-fluoride positron emission tomography (PET)-computed tomography (CT) can detect bioprosthetic aortic valve degeneration and predict valve dysfunction.
Methods: Explanted degenerate bioprosthetic valves were examined ex vivo. Patients with bioprosthetic aortic valves were recruited into 2 cohorts with and without prosthetic valve dysfunction and underwent in vivo contrast-enhanced CT angiography, 18F-fluoride PET, and serial echocardiography during 2 years of follow-up.
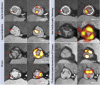
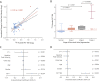
Results: All ex vivo, degenerate bioprosthetic valves displayed 18F-fluoride PET uptake that colocalized with tissue degeneration on histology. In 71 patients without known bioprosthesis dysfunction, 14 had abnormal leaflet pathology on CT, and 24 demonstrated 18F-fluoride PET uptake (target-to-background ratio 1.55 [interquartile range (IQR): 1.44 to 1.88]). Patients with increased 18F-fluoride uptake exhibited more rapid deterioration in valve function compared with those without (annualized change in peak transvalvular velocity 0.30 [IQR: 0.13 to 0.61] vs. 0.01 [IQR: -0.05 to 0.16] ms-1/year; p < 0.001). Indeed 18F-fluoride uptake correlated with deterioration in all the conventional echocardiographic measures of valve function assessed (e.g., change in peak velocity, r = 0.72; p < 0.001). Each of the 10 patients who developed new overt bioprosthesis dysfunction during follow-up had evidence of 18F-fluoride uptake at baseline (target-to-background ratio 1.89 [IQR: 1.46 to 2.59]). On multivariable analysis, 18F-fluoride uptake was the only independent predictor of future bioprosthetic dysfunction.
Conclusions: 18F-fluoride PET-CT identifies subclinical bioprosthetic valve degeneration, providing powerful prediction of subsequent valvular dysfunction and highlighting patients at risk of valve failure. This technique holds major promise in the diagnosis of valvular degeneration and the surveillance of patients with bioprosthetic valves. (18F-Fluoride Assessment of Aortic Bioprosthesis Durability and Outcome [18F-FAABULOUS]; NCT02304276).
Keywords: aortic valve replacement; bioprosthetic valve degeneration; calcification; histology; positron emission tomography.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




Comment in
-
Sodium Fluoride PET and Aortic Bioprosthetic Valve Degeneration: Implications for Patient Diagnosis, Management, and Treatment.J Am Coll Cardiol. 2019 Mar 19;73(10):1120-1122. doi: 10.1016/j.jacc.2018.12.057. J Am Coll Cardiol. 2019. PMID: 30871694 No abstract available.
References
-
- Iung B., Vahanian A. Epidemiology of valvular heart disease in the adult. Nat Rev Cardiol. 2011;8:162–172. - PubMed
-
- Nkomo V.T., Gardin J.M., Skelton T.N., Gottdiener J.S., Scott C.G., Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. - PubMed
-
- Brown J.M., O’Brien S.M., Wu C., Sikora J.A., Griffith B.P., Gammie J.S. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiov Sur. 2009;137:82–90. - PubMed
-
- Pibarot P., Dumesnil J.G. Prosthetic heart valves: selection of the optimal prosthesis and long-term management. Circulation. 2009;119:1034–1048. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- PG/09/083/27667/BHF_/British Heart Foundation/United Kingdom
- G0701127/MRC_/Medical Research Council/United Kingdom
- DH_/Department of Health/United Kingdom
- RE/13/3/30183/BHF_/British Heart Foundation/United Kingdom
- FS/16/19/31982/BHF_/British Heart Foundation/United Kingdom
- WT103782AIA/WT_/Wellcome Trust/United Kingdom
- RM/13/2/30158/BHF_/British Heart Foundation/United Kingdom
- FS/13/77/30488/BHF_/British Heart Foundation/United Kingdom
- FS/14/78/31020/BHF_/British Heart Foundation/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- CH/09/002/BHF_/British Heart Foundation/United Kingdom
- RG/16/10/32375/BHF_/British Heart Foundation/United Kingdom
- FS/12/29/29463/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Medical

