Hippocampal Contributions to Model-Based Planning and Spatial Memory
- PMID: 30871859
- PMCID: PMC6508991
- DOI: 10.1016/j.neuron.2019.02.014
Hippocampal Contributions to Model-Based Planning and Spatial Memory
Abstract
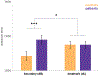
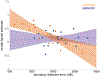
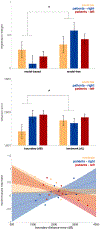
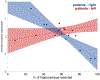
Little is known about the neural mechanisms that allow humans and animals to plan actions using knowledge of task contingencies. Emerging theories hypothesize that it involves the same hippocampal mechanisms that support self-localization and memory for locations. Yet limited direct evidence supports the link between planning and the hippocampal place map. We addressed this by investigating model-based planning and place memory in healthy controls and epilepsy patients treated using unilateral anterior temporal lobectomy with hippocampal resection. Both functions were impaired in the patient group. Specifically, the planning impairment was related to right hippocampal lesion size, controlling for overall lesion size. Furthermore, although planning and boundary-driven place memory covaried in the control group, this relationship was attenuated in patients, consistent with both functions relying on the same structure in the healthy brain. These findings clarify both the neural mechanism of model-based planning and the scope of hippocampal contributions to behavior.
Keywords: anterior temporal lobe; decision-making; hippocampus; human; lesion; memory; model-based; planning; reinforcement learning; spatial.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
No conflicts of interest to declare.
Figures

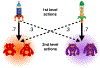
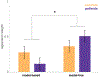
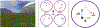
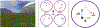
Comment in
-
Hippocampus Is What Happens while You're Busy Making Other Plans.Neuron. 2019 May 8;102(3):517-519. doi: 10.1016/j.neuron.2019.04.027. Neuron. 2019. PMID: 31071284
References
-
- Adams CD (1982). Variations in the sensitivity of instrumental responding to reinforcer devaluation. The Quarterly Journal of Experimental Psychology, 34(2), 77–98.
-
- Adams CD, and Dickinson A (1981). Instrumental responding following reinforcer devaluation. The Quarterly Journal of Experimental Psychology, 33(2), 109–121.
-
- Balleine BW, and Dickinson A (1998). Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology, 37(4), 407–419. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

