Loss of bronchoprotection with ICS plus LABA treatment, β-receptor dynamics, and the effect of alendronate
- PMID: 30872116
- PMCID: PMC6950766
- DOI: 10.1016/j.jaci.2019.01.049
Loss of bronchoprotection with ICS plus LABA treatment, β-receptor dynamics, and the effect of alendronate
Abstract
Background: Loss of bronchoprotection (LOBP) with a regularly used long-acting β2-adrenergic receptor agonist (LABA) is well documented. LOBP has been attributed to β2-adrenergic receptor (B2AR) downregulation, a process requiring farnesylation, which is inhibited by alendronate.
Objective: We sought to determine whether alendronate can reduce LABA-associated LOBP in inhaled corticosteroid (ICS)-treated patients.
Methods: We conducted a randomized, double-blind, placebo-controlled, parallel-design, proof-of-concept trial. Seventy-eight participants with persistent asthma receiving 250 μg of fluticasone twice daily for 2 weeks were randomized to receive alendronate or placebo while initiating salmeterol for 8 weeks. Salmeterol-protected methacholine challenges (SPMChs) and PBMC B2AR numbers (radioligand binding assay) and signaling (cyclic AMP ELISA) were assessed before randomization and after 8 weeks of ICS plus LABA treatment. LOBP was defined as a more than 1 doubling dose reduction in SPMCh PC20 value.
Results: The mean doubling dose reduction in SPMCh PC20 value was 0.50 and 0.27 with alendronate and placebo, respectively (P = .62). Thirty-eight percent of participants receiving alendronate and 33% receiving placebo had LOBP (P = .81). The after/before ICS plus LABA treatment ratio of B2AR number was 1.0 for alendronate (P = .86) and 0.8 for placebo (P = .15; P = .31 for difference between treatments). The B2AR signaling ratio was 0.89 for alendronate (P = .43) and 1.02 for placebo (P = .84; P = .44 for difference). Changes in lung function and B2AR number and signaling were similar between those who did and did not experience LOBP.
Conclusion: This study did not find evidence that alendronate reduces LABA-associated LOBP, which relates to the occurrence of LOBP in only one third of participants. LOBP appears to be less common than presumed in concomitant ICS plus LABA-treated asthmatic patients. B2AR downregulation measured in PBMCs does not appear to reflect LOBP.
Trial registration: ClinicalTrials.gov NCT02230332.
Keywords: bisphosphonate; bronchoprotection; controller therapy; downregulation; loss of bronchoprotection; salmeterol; β(2)-Adrenergic receptor; β(2)-agonists.
Copyright © 2019 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures



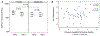
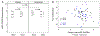
Comment in
-
Bronchoprotective tolerance with inhaled corticosteroid/long-acting β-agonist treatment.J Allergy Clin Immunol. 2019 Sep;144(3):873. doi: 10.1016/j.jaci.2019.05.028. Epub 2019 Jul 9. J Allergy Clin Immunol. 2019. PMID: 31300278 No abstract available.
-
Reply.J Allergy Clin Immunol. 2019 Sep;144(3):873-874. doi: 10.1016/j.jaci.2019.05.029. Epub 2019 Jul 9. J Allergy Clin Immunol. 2019. PMID: 31300279 No abstract available.
References
-
- O’Connor BJ, Aikman SL, Barnes PJ. Tolerance to the nonbronchodilator effects of inhaled beta-2 agonists in asthma. N Engl J Med 1992;327:1204–8. - PubMed
-
- Cockcroft DW, McParland CP, Britto SA, Swystun VA, Rutherford BC. Regular inhaled salbutamol and airway responsiveness to allergen. Lancet 1993; 342:833–7. - PubMed
-
- Cheung D, Timmers MC, Zwinderman AH, Bel EH, Dijkman JH, Sterk PJ. Long-term effects of a long-acting beta-2-adrenoceptor agonist, salmeterol, on airway hyperresponsiveness in patients with mild asthma. N Engl J Med 1992;327: 1198–203. - PubMed
-
- Bhagat R, Kalra S, Swystun VA, Cockcroft DW. Rapid onset of tolerence to the bronchoprotective effect of salmeterol. Chest 1995;108:1235–9. - PubMed
-
- Yates DH, Sussman HS, Shaw MJ, Barnes PJ, Chung KF. Regular formoterol treatment in mild asthma: effect on bronchial responsiveness during and after treatment. Am J Respir Crit Care Med 1995;152:1170–4. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UL1 TR000430/TR/NCATS NIH HHS/United States
- U10 HL098107/HL/NHLBI NIH HHS/United States
- U10 HL098075/HL/NHLBI NIH HHS/United States
- U10 HL098102/HL/NHLBI NIH HHS/United States
- U10 HL098115/HL/NHLBI NIH HHS/United States
- U10 HL098177/HL/NHLBI NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- UL1 TR002003/TR/NCATS NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
- U10 HL098098/HL/NHLBI NIH HHS/United States
- U10 HL064313/HL/NHLBI NIH HHS/United States
- UL1 TR000150/TR/NCATS NIH HHS/United States
- UL1 TR002548/TR/NCATS NIH HHS/United States
- U10 HL098096/HL/NHLBI NIH HHS/United States
- K23 AI125785/AI/NIAID NIH HHS/United States
- T32 HL007118/HL/NHLBI NIH HHS/United States
- UL1 TR000050/TR/NCATS NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- U10 HL098103/HL/NHLBI NIH HHS/United States
- U10 HL098090/HL/NHLBI NIH HHS/United States
- U10 HL098112/HL/NHLBI NIH HHS/United States

