Immunodeficiency and EBV-induced lymphoproliferation caused by 4-1BB deficiency
- PMID: 30872117
- PMCID: PMC6688916
- DOI: 10.1016/j.jaci.2019.03.002
Immunodeficiency and EBV-induced lymphoproliferation caused by 4-1BB deficiency
Abstract
Background: The tumor TNF receptor family member 4-1BB (CD137) is encoded by TNFRSF9 and expressed on activated T cells. 4-1BB provides a costimulatory signal that enhances CD8+ T-cell survival, cytotoxicity, and mitochondrial activity, thereby promoting immunity against viruses and tumors. The ligand for 4-1BB is expressed on antigen-presenting cells and EBV-transformed B cells.
Objective: We investigated the genetic basis of recurrent sinopulmonary infections, persistent EBV viremia, and EBV-induced lymphoproliferation in 2 unrelated patients.
Methods: Whole-exome sequencing, immunoblotting, immunophenotyping, and in vitro assays of lymphocyte and mitochondrial function were performed.
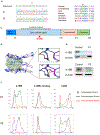
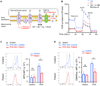
Results: The 2 patients shared a homozygous G109S missense mutation in 4-1BB that abolished protein expression and ligand binding. The patients' CD8+ T cells had reduced proliferation, impaired expression of IFN-γ and perforin, and diminished cytotoxicity against allogeneic and HLA-matched EBV-B cells. Mitochondrial biogenesis, membrane potential, and function were significantly reduced in the patients' activated T cells. An inhibitory antibody against 4-1BB recapitulated the patients' defective CD8+ T-cell activation and cytotoxicity against EBV-infected B cells in vitro.
Conclusion: This novel immunodeficiency demonstrates the critical role of 4-1BB costimulation in host immunity against EBV infection.
Keywords: 4-1BB; CD137; EBV; Hodgkin lymphoma; immunodeficiency.
Copyright © 2019 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


References
-
- Kurth J, Spieker T, Wustrow J, Strickler GJ, Hansmann LM, Rajewsky K, et al. EBV-infected B cells in infectious mononucleosis: viral strategies for spreading in the B cell compartment and establishing latency. Immunity 2000; 13:485–95. - PubMed
-
- Palendira U, Rickinson AB. Primary immunodeficiencies and the control of Epstein-Barr virus infection. Ann N Y Acad Sci 2015; 1356:22–44. - PubMed
-
- Katano H, Ali MA, Patera AC, Catalfamo M, Jaffe ES, Kimura H, et al. Chronic active Epstein-Barr virus infection associated with mutations in perforin that impair its maturation. Blood 2004; 103:1244–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

