Residues in the fingers domain of the translesion DNA polymerase DinB enable its unique participation in error-prone double-strand break repair
- PMID: 30872406
- PMCID: PMC6514613
- DOI: 10.1074/jbc.RA118.006233
Residues in the fingers domain of the translesion DNA polymerase DinB enable its unique participation in error-prone double-strand break repair
Abstract
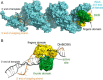
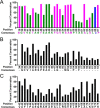
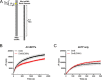
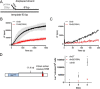
The evolutionarily conserved Escherichia coli translesion DNA polymerase IV (DinB) is one of three enzymes that can bypass potentially deadly DNA lesions on the template strand during DNA replication. Remarkably, however, DinB is the only known translesion DNA polymerase active in RecA-mediated strand exchange during error-prone double-strand break repair. In this process, a single-stranded DNA (ssDNA)-RecA nucleoprotein filament invades homologous dsDNA, pairing the ssDNA with the complementary strand in the dsDNA. When exchange reaches the 3' end of the ssDNA, a DNA polymerase can add nucleotides onto the end, using one strand of dsDNA as a template and displacing the other. It is unknown what makes DinB uniquely capable of participating in this reaction. To explore this topic, we performed molecular modeling of DinB's interactions with the RecA filament during strand exchange, identifying key contacts made with residues in the DinB fingers domain. These residues are highly conserved in DinB, but not in other translesion DNA polymerases. Using a novel FRET-based assay, we found that DinB variants with mutations in these conserved residues are less effective at stabilizing RecA-mediated strand exchange than native DinB. Furthermore, these variants are specifically deficient in strand displacement in the absence of RecA filament. We propose that the amino acid patch of highly conserved residues in DinB-like proteins provides a mechanistic explanation for DinB's function in strand exchange and improves our understanding of recombination by providing evidence that RecA plays a role in facilitating DinB's activity during strand exchange.
Keywords: DNA damage; DNA polymerase; DNA polymerase IV; DNA repair; DNA synthesis; DinB; RecA; homologous recombination.
© 2019 Tashjian et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



References
-
- Friedberg E. C., Walker G. C., Siede W., Wood R. D., Schultz R. A., and Ellenburger T. (2006) DNA Repair and Mutagenesis, ASM Press, Washington, D. C.
-
- Cox M. M. (2007) in Topics in Current Genetics. Molecular Genetics of Recombination. (Aguilera A., and Rothstein R., eds) pp. 53–94, Springer-Verlag, Berlin, Heidelberg, Germany
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

