Efficacy, immunogenicity and safety of a recombinant tetravalent dengue vaccine (CYD-TDV) in children aged 2-17 years: systematic review and meta-analysis
- PMID: 30872537
- PMCID: PMC6429993
- DOI: 10.1136/bmjopen-2017-019368
Efficacy, immunogenicity and safety of a recombinant tetravalent dengue vaccine (CYD-TDV) in children aged 2-17 years: systematic review and meta-analysis
Abstract
Background: Randomised controlled trials have evaluated the recombinant tetravalent dengue vaccine (CYD-TDV). However, individual results may have little power to identify differences among the populations studied.
Objective: To evaluate efficacy, immunogenicity and safety of CYD-TDV in the prevention of dengue in children aged 2-17 years.
Design: Systematic review and meta-analysis.
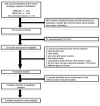
Data sources: MEDLINE (from 1950 to 5 December 2018), EMBASE (from 1947 to 5 December 2018) and Cochrane (from 1993 to 5 December 2018).
Eligibility criteria of studies: Randomised trials comparing efficacy, immunogenicity and safety of CYD-TDV with placebo or other vaccines for preventing dengue cases in children aged 2-17 years.
Outcome measures: Efficacy, immunogenicity and safety of CYD-TDV.
Study appraisal and methods: Calculations were made of relative risk (RR) and mean difference (MD) for dichotomous and continuous outcomes, respectively. All estimates were calculated considering a 95% CI estimate. A p<0.05 was considered statistically significant.
Results: Nine studies involving 34 248 participants were included. The overall efficacy of CYD-TDV was 60% (RR 0.40 (0.30 to 0.54)). Serotype-specific efficacy of the vaccine was 51% for dengue virus type-1 (DENV-1) (RR 0.49 (0.39 to 0.63)); 34% for DENV-2 (RR 0.66 (0.50 to 0.86)); 75% for DENV-3 (RR 0.25 (0.18 to 0.35)) and 77% for DENV-4 (RR 0.23 (0.15 to 0.34)). Overall immunogenicity (MD) of CYD-TDV was 225.13 (190.34 to 259.93). Serotype-specific immunogenicity was: DENV-1: 176.59 (123.36 to 229.83); DENV-2: 294.21 (181.98 to 406.45); DENV-3: 258.78 (146.72 to 370.84) and DENV-4: 189.35 (141.11 to 237.59). The most common adverse events were headache and pain at the injection site.
Limitations: The main limitation of this study was unclear or incomplete data.
Conclusions and implications of key findings: CYD-TDV is considered safe and able to partially protect children and adolescents against four serotypes of DENV for a 1-year period. Despite this, research should prioritise improvements in vaccine efficacy, thus proving higher long-term protection against all virus serotypes.
Prospero registration number: CRD42016043628.
Keywords: child; dengue; vaccine.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
-
- World Health Organization. Dengue: guidelines for diagnosis, treatment, prevention and control. World Health Organization, New edition, 2009. WHO/HTM/NTD/DEN/2009.1 Available at: http://www.who.int/tdr/publications/documents/dengue-diagnosis.pdf/ (accessed 27 Sep 2015). - PubMed
-
- World Health Organization. Guidelines for plaque reduction neutralization testing of human antibodies to dengue viruses. World Health Organization, 2007. WHO/IVB/07.07 Available at: www.who.int/vaccines-documents/ (accessed 15 Jun 2014). - PubMed
-
- World Health Organization. Media Centre. Dengue and severe dengue. Fact sheet N°117, January 2012. Available at: http://www.who.int/mediacentre/factsheets/fs117/en/ (accessed 17 May 2014).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
