Extracellular vesicles in onco-nephrology
- PMID: 30872568
- PMCID: PMC6418250
- DOI: 10.1038/s12276-019-0213-7
Extracellular vesicles in onco-nephrology
Abstract
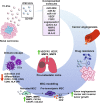
Extracellular vesicles (EVs) are important mediators of intercellular communication in cancer and in normal tissues. EVs transfer biologically active molecules from the cell of origin to recipient cells. This review summarizes the studies on EVs derived from renal cell carcinoma and from a subpopulation of CD105-positive renal cancer stem cells. While EVs from renal cell carcinoma show mild biological activity, EVs from renal cancer stem cells enhance tumor angiogenesis and metastasis formation. The effect is probably due to the transfer of proangiogenic RNA cargo to endothelial cells, which acquire an activated angiogenic phenotype. In vivo, treatment with EVs favors the formation of a premetastatic niche in the lungs. Moreover, EVs derived from renal cancer stem cells modify gene expression in mesenchymal stromal cells, enhancing the expression of genes involved in matrix remodeling, cell migration, and tumor growth. Mesenchymal stromal cells preconditioned with tumor EVs and then coinjected in vivo with renal cancer cells support tumor growth and vessel formation. Finally, tumor EVs promote tumor immune escape by inhibiting the differentiation process of dendritic cells and the activation of T cells. Thus, tumor-derived EVs act on the microenvironment favoring tumor aggressiveness, may contribute to angiogenesis through both direct and indirect mechanisms and are involved in tumor immune escape.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Trajkovic K, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

