Corneal remodelling and topography following biological inlay implantation with combined crosslinking in a rabbit model
- PMID: 30872596
- PMCID: PMC6418097
- DOI: 10.1038/s41598-019-39617-0
Corneal remodelling and topography following biological inlay implantation with combined crosslinking in a rabbit model
Abstract
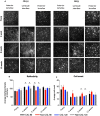
Implantation of biological corneal inlays, derived from small incision lenticule extraction, may be a feasible method for surgical management of refractive and corneal diseases. However, the refractive outcome is dependent on stromal remodelling of both the inlay and recipient stroma. This study aimed to investigate the refractive changes and tissue responses following implantation of 2.5-mm biological inlays with or without corneal collagen crosslinking (CXL) in a rabbit model. Prior to implantation, rotational rheometry demonstrated an almost two-fold increase in corneal stiffness after CXL. After implantation, haze gradually subsided in the CXL-treated inlays (p = 0.001), whereas the untreated inlays preserved their clarity (p = 0.75). In-vivo confocal microscopy revealed reduced keratocyte cell count at the interface of the CXL inlays at week 8. Following initial steepening, regression was observed in anterior mean curvature from week 1 to 12, being most prominent for the non-CXL subgroups (non-CXL: -12.3 ± 2.6D vs CXL: -2.3 ± 4.4D at 90 μm depth, p = 0.03; non-CXL: -12.4 ± 8.0D vs CXL: -5.0 ± 4.0D at 120 μm depth, p = 0.22). Immunohistochemical analysis revealed comparable tissue responses in CXL and untreated subgroups. Our findings suggest that CXL of biological inlays may reduce the time before refractive stabilization, but longer postoperative steroid treatment is necessary in order to reduce postoperative haze.
Conflict of interest statement
Dr. Mehta is a consultant for Ziemer and Carl Zeiss Meditec, Inc. The remaining authors have no conflicts of interests.
Figures




References
-
- Strenk SA, et al. Age-related changes in human ciliary muscle and lens: a magnetic resonance imaging study. Invest. Ophthalmol. Vis. Sci. 1999;40:1162–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

