DNA DSB Repair Dynamics following Irradiation with Laser-Driven Protons at Ultra-High Dose Rates
- PMID: 30872656
- PMCID: PMC6418121
- DOI: 10.1038/s41598-019-40339-6
DNA DSB Repair Dynamics following Irradiation with Laser-Driven Protons at Ultra-High Dose Rates
Abstract
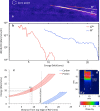
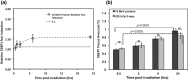
Protontherapy has emerged as more effective in the treatment of certain tumors than photon based therapies. However, significant capital and operational costs make protontherapy less accessible. This has stimulated interest in alternative proton delivery approaches, and in this context the use of laser-based technologies for the generation of ultra-high dose rate ion beams has been proposed as a prospective route. A better understanding of the radiobiological effects at ultra-high dose-rates is important for any future clinical adoption of this technology. In this study, we irradiated human skin fibroblasts-AG01522B cells with laser-accelerated protons at a dose rate of 109 Gy/s, generated using the Gemini laser system at the Rutherford Appleton Laboratory, UK. We studied DNA double strand break (DSB) repair kinetics using the p53 binding protein-1(53BP1) foci formation assay and observed a close similarity in the 53BP1 foci repair kinetics in the cells irradiated with 225 kVp X-rays and ultra- high dose rate protons for the initial time points. At the microdosimetric scale, foci per cell per track values showed a good correlation between the laser and cyclotron-accelerated protons indicating similarity in the DNA DSB induction and repair, independent of the time duration over which the dose was delivered.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Bulanov SV, Esirkepov T, Khoroshkov VS, Kuznetsov AV, Pegoraro F. Oncological hadrontherapy with laser ion accelerators. Phys. Lett. Sect. A Gen. At. Solid State Phys. 2002;299:240–247.
-
- Ledingham KWD, Bolton PR, Shikazono N, Ma C-MC. Towards laser driven hadron cancer radiotherapy: a review of progress. Appl. Sci. 2014;4:402–443. doi: 10.3390/app4030402. - DOI
-
- Macchi A, Borghesi M, Passoni M. Ion acceleration by superintense laser-plasma interaction. Rev. Mod. Phys. 2013;85:751–793. doi: 10.1103/RevModPhys.85.751. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

