Scattering Assisted Imaging
- PMID: 30872736
- PMCID: PMC6418275
- DOI: 10.1038/s41598-019-40997-6
Scattering Assisted Imaging
Abstract
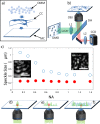
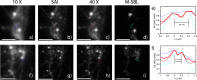
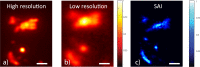
Standard imaging systems provide a spatial resolution that is ultimately dictated by the numerical aperture (NA) of the illumination and collection optics. In biological tissues, the resolution is strongly affected by scattering, which limits the penetration depth to a few tenths of microns. Here, we exploit the properties of speckle patterns embedded into a strongly scattering matrix to illuminate the sample at high spatial frequency content. Combining adaptive optics with a custom deconvolution algorithm, we obtain an increase in the transverse spatial resolution by a factor of 2.5 with respect to the natural diffraction limit. Our Scattering Assisted Imaging (SAI) provides an effective solution to increase the resolution when long working distance optics are needed, potentially paving the way to bulk imaging in turbid tissues.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Abbe E. Beiträge zur theorie des mikroskops und der mikroskopischen wahrnehmung. Arch. für mikroskopische Anat. 1873;9:413–418. doi: 10.1007/BF02956173. - DOI
-
- Rayleigh L. On the theory of optical images, with special reference to the microscope. J. Royal Microsc. Soc. 1903;23:474–482. doi: 10.1111/j.1365-2818.1903.tb04831.x. - DOI
-
- Sheng, P. Introduction to wave scattering, localization and mesoscopic phenomena, vol. 88 (Springer Science & Business Media, 2006).
-
- Vellekoop IM, Lagendijk A, Mosk A. Exploiting disorder for perfect focusing. Nat. photonics. 2010;4:320–322. doi: 10.1038/nphoton.2010.3. - DOI
Publication types
LinkOut - more resources
Full Text Sources

