Age-Related Hearing Loss Is Accelerated by Repeated Short-Duration Loud Sound Stimulation
- PMID: 30872984
- PMCID: PMC6402475
- DOI: 10.3389/fnins.2019.00077
Age-Related Hearing Loss Is Accelerated by Repeated Short-Duration Loud Sound Stimulation
Abstract
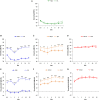
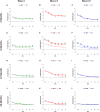
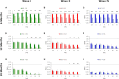
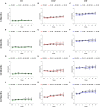
Both age-related hearing loss (ARHL) and noise-induced hearing loss (NIHL) may share pathophysiological mechanisms in that they are associated with excess free radical formation and cochlear blood flow reduction, leading to cochlear damage. Therefore, it is possible that short, but repeated exposures to relatively loud noise during extended time periods, like in leisure (i.e., musical devices and concerts) or occupational noise exposures, may add to cochlear aging mechanisms, having an impact on the onset and/or progression of ARHL. Consequently, the aim of the present study was to determine if repeated short-duration overexposure to a long-term noise could accelerate permanent auditory threshold shifts associated with auditory aging in an animal model of ARHL. Toward this goal, young adult, 3-month-old Wistar rats were divided into two groups: one exposed (E) and the other non-exposed (NE) to noise overstimulation. The stimulation protocol consisted of 1 h continuous white noise at 110 dB sound pressure level (SPL), 5 days a week, allowing 2 days for threshold recovery before initiating another stimulation round, until the animals reached an age of 18 months. Auditory brainstem response (ABR) recordings at 0.5, 1, 2, 4, 8, 16, and 32 kHz were performed at 3, 6, 12, and 18 months of age. The results demonstrate that in the E group there were significant increases in auditory thresholds at all tested frequencies starting already at 6 months of age, which extended at 12 and 18 months. However, in NE animals threshold shifts were not evident until 12 months, extending to 18 months of age. Threshold shifts observed in the E animals at 6 and 12 months were significantly larger than those observed in the NE group at the same ages. Threshold shifts at 6 and 12 months in E animals resembled those at 12 and 18 months in NE animals, respectively. This suggests that repeated noise overstimulation in short-duration episodes accelerates the time-course of hearing loss in this animal model of ARHL.
Keywords: auditory brainstem responses; evoked potentials; noise-induced hearing loss; presbycusis; sensorineural hearing loss.
Figures



References
-
- Alvarado J. C., Fuentes-Santamaría V., Gabaldón-Ull M. C., Jareño-Flores T., Miller J. M., Juiz J. M. (2016). Noise-induced “Toughening” effect in wistar rats: enhanced auditory brainstem responses are related to calretinin and nitric oxide synthase upregulation. Front. Neuroanat. 10:19 10.3389/fnana.2016.00019 - DOI - PMC - PubMed
-
- Alvarado J. C., Fuentes-Santamaría V., Melgar-Rojas P., Valero M. L., Gabaldón-Ull M. C., Miller J. M., et al. (2015). Synergistic effects of free radical scavengers and cochlear vasodilators: a new otoprotective strategy for age-related hearing loss. Front. Aging Neurosci. 7:86. 10.3389/fnagi.2015.00086 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

