Emerging Therapeutic Approaches for Cystic Fibrosis. From Gene Editing to Personalized Medicine
- PMID: 30873022
- PMCID: PMC6400831
- DOI: 10.3389/fphar.2019.00121
Emerging Therapeutic Approaches for Cystic Fibrosis. From Gene Editing to Personalized Medicine
Abstract
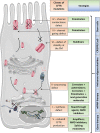
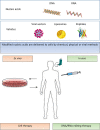
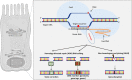
An improved understanding of the cystic fibrosis (CF) transmembrane conductance regulator (CFTR) protein structure and the consequences of CFTR gene mutations have allowed the development of novel therapies targeting specific defects underlying CF. Some strategies are mutation specific and have already reached clinical development; some strategies include a read-through of the specific premature termination codons (read-through therapies, nonsense mediated decay pathway inhibitors for Class I mutations); correction of CFTR folding and trafficking to the apical plasma membrane (correctors for Class II mutations); and an increase in the function of CFTR channel (potentiators therapy for Class III mutations and any mutant with a residual function located at the membrane). Other therapies that are in preclinical development are not mutation specific and include gene therapy to edit the genome and stem cell therapy to repair the airway tissue. These strategies that are directed at the basic CF defects are now revolutionizing the treatment for patients and should positively impact their survival rates.
Keywords: CFTR; CFTR modulator; cystic fibrosis; gene therapy; ivacaftor.
Figures
References
-
- Alton E. W. F. W., Armstrong D. K., Ashby D., Bayfield K. J., Bilton D., Bloomfield E. V., et al. (2015). Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir. Med. 3 684–691. 10.1016/S2213-2600(15)00245-3 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

